Horse Liver Alcohol Dehydrogenase
From Proteopedia
| Line 11: | Line 11: | ||
== Protein Structure == | == Protein Structure == | ||
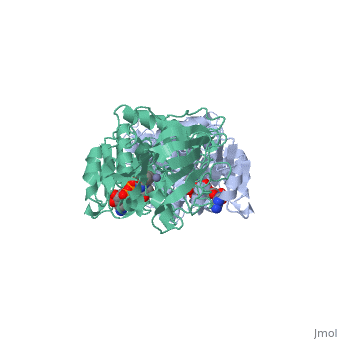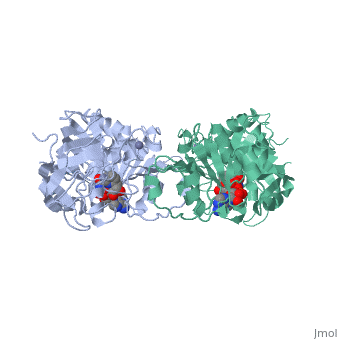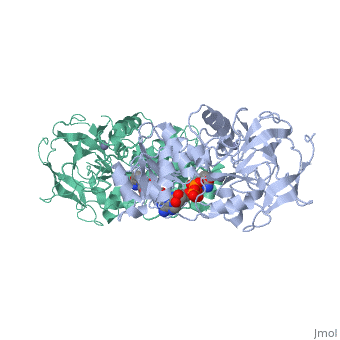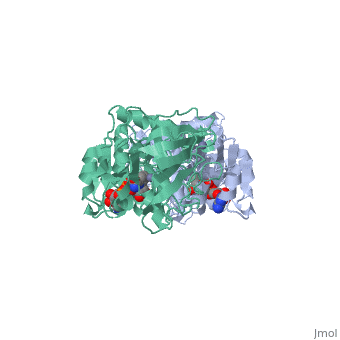
| - | Liver alcohol dehydrogenase (LADH | + | Liver alcohol dehydrogenase (LADH) is a homodimeric protein. Each monomer contains 374 amino acids and has a mass of 40 kDa, with each monomer also containing a catalytic and coenzyme binding domain. The coenzyme-binding domains have structures similar to those found in several other NAD-dependent dehydrogenases (Rossmann et al., 1973---structure), and they form a 12-stranded beta-pleated sheet structure that makes up the central core of the dimer (Eklund et al., 1976---structure). |
| - | + | ||
| + | Through crystal structure analysis of horse liver alcohol dehydrogenase, which has been extended to 2.4 Å resolution, electron density maps of the apoenzyme have determined the positions of the 374 amino acids in the polypeptide chain of each subunit (Hans Eklund). | ||
| + | |||
| + | The coenzyme binding domain of the subunit comprises residues 176 to 318. Fourty-five percent of these residues are helical and 32% are in the central six-stranded pleated sheet structure(Hans Eklund).The positions and orientations of the helices with respect to the pleated sheet indicate a possible folding mechanism for this part of the subunit structure. (Hans Eklund). | ||
| + | |||
| + | The catalytic domain is mainly built up from three distinct antiparallel pleated-sheet regions. Residues within this domain provide ligands to the catalytic <scene name='Sandbox_163/Zinc/1'>zinc</scene> atom; Cys46, His67 and Cys174(zinc). An approximate tetrahedral coordination of this zinc is completed by a water molecule or hydroxyl ion depending on the pH. Residues 95 to 113 form a lobe that binds the second zinc atom of the subunit. This zinc is liganded in a distorted tetrahedral arrangement by four sulphur atoms from the cysteine residues 97, 100, 103 and 111. The lobe forms one side of a significant cleft in the enzyme surface suggesting that this region might constitute a second catalytic centre of unknown function.(Zinc) | ||
| + | |||
| + | The two domains of the subunit are separated by a crevice that contains a wide and deep hydrophobic pocket. The catalytic zinc atom is at the bottom of this pocket, with the zinc-bound water molecule projecting out into the pocket. This water molecule is hydrogen-bonded to the side chain of Ser48 which in turn is hydrogen-bonded to His51. The pocket which in all probability is the binding site for the substrate and the nicotinamide moiety of the coenzyme, is lined almost exclusively with hydrophobic side chains.(Active). Both subunits contribute residues to each of the two substrate binding pockets of the molecule. The only accessible polar groups in the vicinity of the catalytic centre are Ser48 and Thr178 apart from zinc and the zinc-bound water molecule(Zinc). | ||
From structural determinants it by x-ray diffraction it is known that LADH undergoes conformational changes going from an open structure, in which large binding areas for coenzymes and substrates are accessible, towards a closed conformation. However the trigger mechanism for the structural transition is not yet fully understood. A complex pattern has emerged, showing that the structural changes in the LADH depend on coenzyme analogue structure and on which combination of coenzyme and a second ligand is present. | From structural determinants it by x-ray diffraction it is known that LADH undergoes conformational changes going from an open structure, in which large binding areas for coenzymes and substrates are accessible, towards a closed conformation. However the trigger mechanism for the structural transition is not yet fully understood. A complex pattern has emerged, showing that the structural changes in the LADH depend on coenzyme analogue structure and on which combination of coenzyme and a second ligand is present. | ||
Revision as of 22:50, 30 March 2010
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Contents |
Horse Liver Alcohol Dehydrogenase
Meghan Hatcher
General Information
Alcohol dehydrogenase (LADH) is found in the liver of the species Equus Caballus (horse). It is a dimeric zinc-dependant protein, that catalyzes the reversible oxidation of primary and secondary alcohols to aldehyde, requiring the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD).
|
Protein Structure
Liver alcohol dehydrogenase (LADH) is a homodimeric protein. Each monomer contains 374 amino acids and has a mass of 40 kDa, with each monomer also containing a catalytic and coenzyme binding domain. The coenzyme-binding domains have structures similar to those found in several other NAD-dependent dehydrogenases (Rossmann et al., 1973---structure), and they form a 12-stranded beta-pleated sheet structure that makes up the central core of the dimer (Eklund et al., 1976---structure).
Through crystal structure analysis of horse liver alcohol dehydrogenase, which has been extended to 2.4 Å resolution, electron density maps of the apoenzyme have determined the positions of the 374 amino acids in the polypeptide chain of each subunit (Hans Eklund).
The coenzyme binding domain of the subunit comprises residues 176 to 318. Fourty-five percent of these residues are helical and 32% are in the central six-stranded pleated sheet structure(Hans Eklund).The positions and orientations of the helices with respect to the pleated sheet indicate a possible folding mechanism for this part of the subunit structure. (Hans Eklund).
The catalytic domain is mainly built up from three distinct antiparallel pleated-sheet regions. Residues within this domain provide ligands to the catalytic atom; Cys46, His67 and Cys174(zinc). An approximate tetrahedral coordination of this zinc is completed by a water molecule or hydroxyl ion depending on the pH. Residues 95 to 113 form a lobe that binds the second zinc atom of the subunit. This zinc is liganded in a distorted tetrahedral arrangement by four sulphur atoms from the cysteine residues 97, 100, 103 and 111. The lobe forms one side of a significant cleft in the enzyme surface suggesting that this region might constitute a second catalytic centre of unknown function.(Zinc)
The two domains of the subunit are separated by a crevice that contains a wide and deep hydrophobic pocket. The catalytic zinc atom is at the bottom of this pocket, with the zinc-bound water molecule projecting out into the pocket. This water molecule is hydrogen-bonded to the side chain of Ser48 which in turn is hydrogen-bonded to His51. The pocket which in all probability is the binding site for the substrate and the nicotinamide moiety of the coenzyme, is lined almost exclusively with hydrophobic side chains.(Active). Both subunits contribute residues to each of the two substrate binding pockets of the molecule. The only accessible polar groups in the vicinity of the catalytic centre are Ser48 and Thr178 apart from zinc and the zinc-bound water molecule(Zinc).
From structural determinants it by x-ray diffraction it is known that LADH undergoes conformational changes going from an open structure, in which large binding areas for coenzymes and substrates are accessible, towards a closed conformation. However the trigger mechanism for the structural transition is not yet fully understood. A complex pattern has emerged, showing that the structural changes in the LADH depend on coenzyme analogue structure and on which combination of coenzyme and a second ligand is present.
Protein Function
LADH catalyzes the transfer of hydride ion from an alcohol substrate to the NAD+ cofactor, yielding an aldehyde and the reduced cofactor, NADH. X-ray crystallography has shown that the NAD+ binds in an extended conformation with 15 Å between the buried, reacting carbon of the nicotinamide ring and the adenine ring near the surface of the protein (*). Although the second order reaction of wild-type enzyme with benzyl alcohol is partially rate-limited by aldehyde product release, it has been possible to make the chemistry more rate limiting with only a single, active-site mutation.
Proteopedia Page Contributors and Editors (what is this?)
Meghan Hatcher, Alexander Berchansky, David Canner, Michal Harel, Simmi Parhar, Andrea Gorrell, Eran Hodis