Sandbox 171
From Proteopedia
| Line 3: | Line 3: | ||
''' | ''' | ||
{{STRUCTURE_2mys| PDB=2mys| Scene =Sandbox_171/Newscene/1'>active site }} | {{STRUCTURE_2mys| PDB=2mys| Scene =Sandbox_171/Newscene/1'>active site }} | ||
| - | + | Myosin | |
==Overview== | ==Overview== | ||
Myosin is one of three major classes of molecular motors: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. <ref name="Spudich">PMID: 8824453 </ref> | Myosin is one of three major classes of molecular motors: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. <ref name="Spudich">PMID: 8824453 </ref> | ||
| Line 20: | Line 20: | ||
| - | + | ==Literature Cited== | |
<references/> | <references/> | ||
Revision as of 04:16, 1 April 2010
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. | |||||||||
| |||||||||
| 2mys, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Myosin
Contents |
Overview
Myosin is one of three major classes of molecular motors: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. [1]
Structure
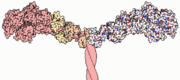
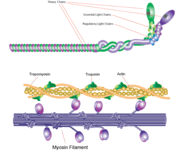
Myosin has a molecular size of approximately 520 kilodaltons, with two 220 kD heavy chains and two pairs of light chains which vary in size. [2] The molecule is asymmetric, having a long tail and two globular heads. [2] Each heavy chains composes the bulk of one of the globular heads. [2] Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.[2] Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. [2]
Function
Literature Cited
- ↑ Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb Symp Quant Biol. 1995;60:783-91. PMID:8824453
- ↑ 2.0 2.1 2.2 2.3 2.4 Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857