Sandbox 171
From Proteopedia
| Line 5: | Line 5: | ||
{{STRUCTURE_2mys| PDB=2mys| Scene =Sandbox_171/Newscene/1'>active site }} | {{STRUCTURE_2mys| PDB=2mys| Scene =Sandbox_171/Newscene/1'>active site }} | ||
==Overview== | ==Overview== | ||
| - | Myosin is one of three major classes of molecular | + | Myosin is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. <ref name="Spudich">PMID: 8824453 </ref> |
===Crystallization and X-ray diffraction=== | ===Crystallization and X-ray diffraction=== | ||
| - | Myosin is found in abundance, therefore can be prepared in gram quantities. <ref name="Rayment">PMID: 8316857</ref> For nearly 30 years the myosin head was resistant to crystallization yet by 1993 researchers discovered a mechanism to obtain x-ray quality crystals. <ref name="Rayment" /> The process modified the protein by reductive methylation. <ref name="Rayment" /> X-ray data was used to determine the structure. <ref name="Rayment" /> | + | Myosin is found in abundance, therefore it can be prepared in gram quantities. <ref name="Rayment">PMID: 8316857</ref> For nearly 30 years the myosin head was resistant to crystallization yet by 1993 researchers discovered a mechanism to obtain x-ray quality crystals. <ref name="Rayment" /> The process modified the protein by reductive methylation. <ref name="Rayment" /> X-ray data was used to determine the tertiary structure of the protein. <ref name="Rayment" /> |
==Structure== | ==Structure== | ||
| + | [[Image:Myosin_head.gif|thumb|alt=Alt text|Myosin head]] | ||
| + | [[Image:Myosin_chains.gif|thumb|alt=Alt text|Myosin filament]] | ||
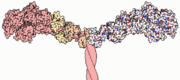
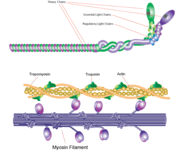
Myosin has a molecular size of approximately 520 kilodaltons, with two 220 kD heavy chains and two pairs of light chains which vary in size.<ref name="Rayment" /> The molecule is asymmetric, having a long tail and two globular heads. <ref name="Rayment" /> Each heavy chains composes the bulk of one of the globular heads. <ref name="Rayment" /> Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.<ref name="Rayment" /> Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. <ref name="Rayment" /> | Myosin has a molecular size of approximately 520 kilodaltons, with two 220 kD heavy chains and two pairs of light chains which vary in size.<ref name="Rayment" /> The molecule is asymmetric, having a long tail and two globular heads. <ref name="Rayment" /> Each heavy chains composes the bulk of one of the globular heads. <ref name="Rayment" /> Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.<ref name="Rayment" /> Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. <ref name="Rayment" /> | ||
| + | |||
The myosin head is assymetrical with a length of 165 Angstroms and 65 Angstroms in width, with a total thickness of about 40 Angstroms. <ref name="Rayment" /> About 48% of the amino acid residues in the myosin head are dominated by α helices. <ref name="Rayment" /> One long α helix of about 85 Angstroms stretches from the thick part of the myosin head to the COOH-terminus of the heavy chain. <ref name="Rayment" /> This particular helix forms the light chain binding region on the heavy chain. <ref name="Rayment" /> | The myosin head is assymetrical with a length of 165 Angstroms and 65 Angstroms in width, with a total thickness of about 40 Angstroms. <ref name="Rayment" /> About 48% of the amino acid residues in the myosin head are dominated by α helices. <ref name="Rayment" /> One long α helix of about 85 Angstroms stretches from the thick part of the myosin head to the COOH-terminus of the heavy chain. <ref name="Rayment" /> This particular helix forms the light chain binding region on the heavy chain. <ref name="Rayment" /> | ||
| - | [[Image:Myosin_head.gif|thumb|alt=Alt text|Myosin head]] | ||
| - | [[Image:Myosin_chains.gif|thumb|alt=Alt text|Myosin filament]] | ||
==Function== | ==Function== | ||
| + | |||
Click the link to access DNAtube video "A Moving Myosin Motor Protein" | Click the link to access DNAtube video "A Moving Myosin Motor Protein" | ||
http://www.dnatube.com/video/389/A-Moving-Myosin-Motor-Protein-myosin-actin-interaction | http://www.dnatube.com/video/389/A-Moving-Myosin-Motor-Protein-myosin-actin-interaction | ||
Revision as of 04:46, 1 April 2010
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. | |||||||||
| |||||||||
| 2mys, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Overview
Myosin is one of three major classes of molecular motor proteins: myosin, dynein, and kinesin. As the most abundant of these proteins myosin plays a structural and enzymatic role in muscle contraction and intracellular motility. Myosin was first discovered in muscle in the 19th century. [1]
Crystallization and X-ray diffraction
Myosin is found in abundance, therefore it can be prepared in gram quantities. [2] For nearly 30 years the myosin head was resistant to crystallization yet by 1993 researchers discovered a mechanism to obtain x-ray quality crystals. [2] The process modified the protein by reductive methylation. [2] X-ray data was used to determine the tertiary structure of the protein. [2]
Structure
Myosin has a molecular size of approximately 520 kilodaltons, with two 220 kD heavy chains and two pairs of light chains which vary in size.[2] The molecule is asymmetric, having a long tail and two globular heads. [2] Each heavy chains composes the bulk of one of the globular heads. [2] Subfragment-1(S1) also termed the myosin head consists of ATP, actin, and two light chain binding sites.[2] Each globular head has a heavy chain and two light chains for a combined molecular size of about 130 kD. [2]
The myosin head is assymetrical with a length of 165 Angstroms and 65 Angstroms in width, with a total thickness of about 40 Angstroms. [2] About 48% of the amino acid residues in the myosin head are dominated by α helices. [2] One long α helix of about 85 Angstroms stretches from the thick part of the myosin head to the COOH-terminus of the heavy chain. [2] This particular helix forms the light chain binding region on the heavy chain. [2]
Function
Click the link to access DNAtube video "A Moving Myosin Motor Protein" http://www.dnatube.com/video/389/A-Moving-Myosin-Motor-Protein-myosin-actin-interaction
Literature Cited
- ↑ Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb Symp Quant Biol. 1995;60:783-91. PMID:8824453
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50-8. PMID:8316857