Ketosteroid Isomerase
From Proteopedia
| Line 14: | Line 14: | ||
Although the <scene name='User:Laura_M._Haynes/Sandbox_1/Active_site_1isk/1'>active site</scene> of KSI is notably hydrophobic, it contains several hydrophilic residues believed to be important to the enzymatic function of the protein. The hydrophobic active site of KSI contains an aspartate residue at position 99 and a tyrosine residue at position 14 (according to the numbering for the ''Commamonas tetosteroni'' protein, which will be used throughout) that are capable of binding the 3-position carbonyl of the steroid. Additionally, the active site contains an aspartate residue at position 38 that is participates in the catalytic activity of KSI.<ref name="Pollack" /> | Although the <scene name='User:Laura_M._Haynes/Sandbox_1/Active_site_1isk/1'>active site</scene> of KSI is notably hydrophobic, it contains several hydrophilic residues believed to be important to the enzymatic function of the protein. The hydrophobic active site of KSI contains an aspartate residue at position 99 and a tyrosine residue at position 14 (according to the numbering for the ''Commamonas tetosteroni'' protein, which will be used throughout) that are capable of binding the 3-position carbonyl of the steroid. Additionally, the active site contains an aspartate residue at position 38 that is participates in the catalytic activity of KSI.<ref name="Pollack" /> | ||
| + | |||
==Enzyme Mechanism== | ==Enzyme Mechanism== | ||
| - | <applet load='1QJG' size='400' frame='true' align='left' caption='KSI complexed with | + | <applet load='1QJG' size='400' frame='true' align='left' caption='KSI complexed with equinelin (1QJG)' scene='User:Laura_M._Haynes/Sandbox_1/1qjg/1'/> |
| + | ===General Mechanism=== | ||
The general mechanism of the proposed reaction of ketosteroid isomerase involves the breaking of a C-H bond adjacent to a carbonyl. This is typically regarded as a difficult reaction due to instability of the intermediate; however it is observed in a number of enzyme-mediated biological reactions.<ref name="Pollack" /> In line with other biological reactions, the mechanism of KSI involves the abstraction the β-hyrdogen from the 4-position carbon resulting in the formation of an enol intermediate, which is followed by reketonization.<ref name="Pollack" />,<ref name="Ha" /> Structural and kinetic studies suggest that Asp<sup>38</sup> (numbering is that of the TI varient of KSI) serves as a general base in this reaction as shown below. Note the formation of the unstable enolate intermediate. | The general mechanism of the proposed reaction of ketosteroid isomerase involves the breaking of a C-H bond adjacent to a carbonyl. This is typically regarded as a difficult reaction due to instability of the intermediate; however it is observed in a number of enzyme-mediated biological reactions.<ref name="Pollack" /> In line with other biological reactions, the mechanism of KSI involves the abstraction the β-hyrdogen from the 4-position carbon resulting in the formation of an enol intermediate, which is followed by reketonization.<ref name="Pollack" />,<ref name="Ha" /> Structural and kinetic studies suggest that Asp<sup>38</sup> (numbering is that of the TI varient of KSI) serves as a general base in this reaction as shown below. Note the formation of the unstable enolate intermediate. | ||
[[Image:General_base.jpg|center]] | [[Image:General_base.jpg|center]] | ||
| - | Tyr<sup>14</sup> and Asp<sup>99</sup> are believed to participate in hydrogen bonding to the O-3 carbonyl of the substrate steroid and stabilize reaction intermediates. Tyr<sup>14</sup> is also believed to participate in a low barrier hydrogen bond with the 3-postition oxygen of the steroid, thereby facilitating the abstraction of the β-hydrogen at the 4-position. There are two proposed models of this hydrogen bonding. In <scene name='User:Laura_M._Haynes/Sandbox_1/Model_1_1qjg/1'>Model 1</scene>, Tyr<sup>14</sup> and Asp<sup>99</sup> are both bound to the 3-position oxygen, whereas, in <scene name='User:Laura_M._Haynes/Sandbox_1/Model_2_1qjg/1'>Model 2</scene>, they form a hydrogen bonding network. These are shown here with the intermediate analog equilenin.<ref name="Cho2">PMID:10551849</ref> | + | Tyr<sup>14</sup> and Asp<sup>99</sup> are believed to participate in hydrogen bonding to the O-3 carbonyl of the substrate steroid and stabilize reaction intermediates. Tyr<sup>14</sup> is also believed to participate in a low barrier hydrogen bond with the 3-postition oxygen of the steroid, thereby facilitating the abstraction of the β-hydrogen at the 4-position. There are two proposed models of this hydrogen bonding. In <scene name='User:Laura_M._Haynes/Sandbox_1/Model_1_1qjg/1'>Model 1</scene>, Tyr<sup>14</sup> and Asp<sup>99</sup> are both bound to the 3-position oxygen, whereas, in <scene name='User:Laura_M._Haynes/Sandbox_1/Model_2_1qjg/1'>Model 2</scene>, they form a hydrogen bonding network. These are <scene name='User:Laura_M._Haynes/Sandbox_1/1qjg/1'>shown here</scene> with the intermediate analog equilenin.<ref name="Cho2">PMID:10551849</ref> |
| + | |||
| + | ===Stereochemistry=== | ||
| + | |||
==Related Proteins== | ==Related Proteins== | ||
Revision as of 20:58, 3 April 2010
Contents |
Ketosteroid Isomerase
Introduction
|
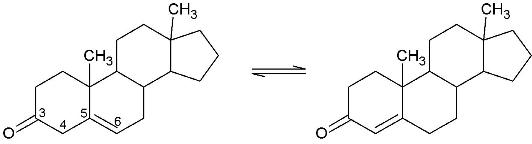
(KSI, EC#5.3.3.1) is an enzyme that catalyzes the isomerization of 3-oxo-Δ5 ketosteroids to their hormonally active Δ4-conjugated isomers, as illustrated below.[1], [2]
This reaction is essential in the biosynthesis of steroids in mammals where KSI is a membrane-bound complex.[3] In bacteria, however, KSI exists as a soluble protein is involves in catabolism of steroids.[3] It was first isolated in and has been extensively studied in Commamonas tetosteroni (TI), a bacteria that is capable of It is one of the most efficient known enzymes with an essentially diffusion limited rate of catalysis.[2]
An NMR solution phase structure of KSI was solved in 1997 by Wu et al.[4] allowing greater insight into the mechanism of this intriguing enzyme.
Structure
Ketosteroid isomerase exits as a 28 kDa homodimeric protein, in which the two dimers related to each other via hydrophobic and electrostatic interactions.[4] Each dimer consists of a curved and three . These secondary structures define a conical closed barrel geometry, with one open and one closed end, and create a deep pocket in which the active site resides.[3],[5] This unique geometry is shared by several other proteins (scytalone dehydratase, nuclear transport factor 2, and naphthalene 1,2-dioxygenase), however, these molecules do not share functional or sequence homology. It is speculated that this unique protein structure may enable better binding of hydrophobic substrates such as steroids.[3]
Although the of KSI is notably hydrophobic, it contains several hydrophilic residues believed to be important to the enzymatic function of the protein. The hydrophobic active site of KSI contains an aspartate residue at position 99 and a tyrosine residue at position 14 (according to the numbering for the Commamonas tetosteroni protein, which will be used throughout) that are capable of binding the 3-position carbonyl of the steroid. Additionally, the active site contains an aspartate residue at position 38 that is participates in the catalytic activity of KSI.[1]
Enzyme Mechanism
|
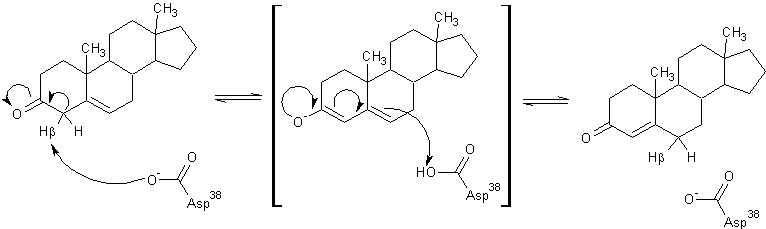
General Mechanism
The general mechanism of the proposed reaction of ketosteroid isomerase involves the breaking of a C-H bond adjacent to a carbonyl. This is typically regarded as a difficult reaction due to instability of the intermediate; however it is observed in a number of enzyme-mediated biological reactions.[1] In line with other biological reactions, the mechanism of KSI involves the abstraction the β-hyrdogen from the 4-position carbon resulting in the formation of an enol intermediate, which is followed by reketonization.[1],[3] Structural and kinetic studies suggest that Asp38 (numbering is that of the TI varient of KSI) serves as a general base in this reaction as shown below. Note the formation of the unstable enolate intermediate.
Tyr14 and Asp99 are believed to participate in hydrogen bonding to the O-3 carbonyl of the substrate steroid and stabilize reaction intermediates. Tyr14 is also believed to participate in a low barrier hydrogen bond with the 3-postition oxygen of the steroid, thereby facilitating the abstraction of the β-hydrogen at the 4-position. There are two proposed models of this hydrogen bonding. In , Tyr14 and Asp99 are both bound to the 3-position oxygen, whereas, in , they form a hydrogen bonding network. These are with the intermediate analog equilenin.[6]
Stereochemistry
Related Proteins
Available Structures
References
- ↑ 1.0 1.1 1.2 1.3 Pollack RM. Enzymatic mechanisms for catalysis of enolization: ketosteroid isomerase. Bioorg Chem. 2004 Oct;32(5):341-53. PMID:15381400 doi:10.1016/j.bioorg.2004.06.005
- ↑ 2.0 2.1 TALALAY P, WANG VS. Enzymic isomerization of delta5-3-ketosteroids. Biochim Biophys Acta. 1955 Oct;18(2):300-1. PMID:13276386
- ↑ 3.0 3.1 3.2 3.3 3.4 Ha NC, Choi G, Choi KY, Oh BH. Structure and enzymology of Delta5-3-ketosteroid isomerase. Curr Opin Struct Biol. 2001 Dec;11(6):674-8. PMID:11751047
- ↑ 4.0 4.1 Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Solution structure of 3-oxo-delta5-steroid isomerase. Science. 1997 Apr 18;276(5311):415-8. PMID:9103200
- ↑ Cho HS, Choi G, Choi KY, Oh BH. Crystal structure and enzyme mechanism of Delta 5-3-ketosteroid isomerase from Pseudomonas testosteroni. Biochemistry. 1998 Jun 9;37(23):8325-30. PMID:9622484 doi:10.1021/bi9801614
- ↑ Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH. Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization. J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:10551849
Proteopedia Page Contributors and Editors (what is this?)
Laura M. Haynes, Michal Harel, Joel L. Sussman, Alexander Berchansky