Joel L. Sussman Sandbox 1
From Proteopedia
| Line 25: | Line 25: | ||
BChE+organophosphate inhibitors causing irreversible inhibition | BChE+organophosphate inhibitors causing irreversible inhibition | ||
| - | |||
| - | ==Background== | ||
| - | <table width='300' align='right' cellpadding='5'><tr><td rowspan='2'> </td><td bgcolor='#eeeeee'><applet load='1bl8' size='290' frame='true' align='right' scene='Cation-pi_interactions/1bl8_pi2/3' /></td></tr><tr><td bgcolor='#eeeeee'><center>'''Cation-pi interactions''' (<scene name='Cation-pi_interactions/1bl8_pi2/3'>initial scene</scene>).</center></td></tr></table> | ||
| - | Gallivan and Dougherty (1999)<ref name='GD' /> reported results from a quantitative survey of cation-pi interactions in high-resolution structures in the Protein Data Bank. Using an energy-based criterion for identifying significant sidechain interactions, they studied 593 sequence dissimilar proteins. They found an average of one such interaction per 77 residues, with no significant effect of chain length, or multiple-chain vs. single chain structures. Arg was more likely than Lys to participate in a cation-pi interaction, and the likelihood of aromatic sidechain participation was Trp > Tyr > Phe. Over one quarter of all Trp's were involved in cation-pi interactions, with the cation typically positioned over the 6-atom ring of Trp. Because of the frequencies of amino acids in the database, Arg participates in nearly twice as many cation-pi interactions as does Lys, and the numbers of cation-pi interactions involving Trp, Tyr and Phe are roughly similar. Their study did not include His because, depending on its protonation state, it could participate either as a cation or as a pi-system. Lys and Arg were assumed always to be protonated and hence cationic. | ||
| - | |||
| - | Gallivan and Dougherty conclude "When a cationic sidechain is near an aromatic sidechain, the geometry is biased toward one that would experience a favorable cation-pi interaction", and "cation-pi interactions should be considered alongside the more conventional hydrogen bonds, salt bridges, and hydrophobic effects in any analysis of protein structure". They provide a [http://capture.caltech.edu/a server that lists text results from their program CaPTURE]. | ||
| - | |||
| - | Zacharias and Dougherty (2002)<ref>PMID: 12084634</ref> reviewed cation-pi interactions in the binding of ligands to proteins. Cation-pi interactions are usually energetically important when the ligand has either positive charge or an aromatic ring, and are involved in control of ion channels, G-protein-coupled receptors, transporters, and enzymatic catalysis. An example is [[1l8b]], <scene name='Cation-pi_interactions/1l8b_pi5/1'>a portion of a eukaryotic translation initiation factor that recognizes N7-methylated guanosine on the 5'-end of mRNAs. The ligand's heterocyclic base (cationic) is sandwiched between Trp56 and Trp102</scene>. | ||
| - | |||
| - | ==Examples== | ||
| - | <table width='300' align='right' cellpadding='5'><tr><td rowspan='2'> </td><td bgcolor='#eeeeee'><applet load='1gai' size='290' frame='true' align='right' scene='Cation-pi_interactions/1gai_pi4/1' /></td></tr><tr><td bgcolor='#eeeeee'><center>'''Cation-pi interactions''' (<scene name='Cation-pi_interactions/1gai_pi4/1'>initial scene</scene>).</center></td></tr></table> | ||
| - | Examples given by Gallavan and Dougherty<ref name='GD' /> include: | ||
| - | |||
| - | *[[1gai]]: <scene name='Cation-pi_interactions/1gai_pi4/1'>a 472-amino acid chain (glucoamylase) with a spectacular cluster of four aromatic rings (two Trp's, two Tyr's) around a single Lys108</scene>. | ||
| - | |||
| - | *[[1bfg]]: a 126-amino acid chain (fibroblast growth factor) with an unusually high incidence of cation-pi interactions. Gallivan and Dougherty report 5 energetically significant interactions. | ||
| - | |||
| - | *[[2wea]]: the longest single chain (323 residues) with no energetically significant cation-pi interactions from the original set of 593 proteins originally analyzed. | ||
| - | |||
| - | Interesting examples noted by [[User:Eric_Martz| Eric Martz]]: | ||
| - | |||
| - | *[[1axi]] (human growth hormone): Chain B contains an unusual string of three aromatic sidechains separated by, and capped at the ends with, 4 cationic sidechains. (Using "c" for cation and "p" for pi, the chain is "cpcpcpc".) Only the one of these six interactions is deemed energetically insignificant by CaPTURE (Lys at one end). See image [http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/cp-1axi.gif here]. | ||
| - | *[[1bl8]] <scene name='Cation-pi_interactions/1bl8_pi2/3'>(bacterial potassium channel): There is a single interchain cation-pi pair for each contact between chains of this homotetramer, but no intrachain cation-pi interactions.</scene> | ||
| - | *[[2vab]] (peptide bound to class I histocompatibility protein): The N-terminal Phe of the nonapeptide stacks with Trp 167 of the protein. On either side of the stacked rings are cations (Arg 170, Lys 66), forming the unusual cppc chain RWFK. See image [http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/cp-2vab.gif here]. | ||
| - | *[[1rog]] (peptide bound to class I histocompatibility protein): Three (of the four) cations in the 9-residue peptide interact with aromatic sidechains in the protein groove. This is a theoretical model. | ||
| - | *[[1dlh]] (peptide bound to class II histocompatibility protein): There are no cation-pi interactions for the 13-residue peptide, despite its containing three lysines and one tyrosine. A number of nearby sidechains that potentially could interact appear to be blocked by other noncovalent bonding interactions, and the peptide lysine sidechains are generally pointing away from the protein. | ||
| - | |||
| - | ==Additional Related structures== | ||
| - | *[[AChE_inhibitors_and_substrates_(Part_II)]] | ||
| - | *[[:Category:Cation-pi|Entries in Proteopedia's Cation-pi Category]] (with hyphen) | ||
| - | *[[:Category:Cation_pi|Entries in Proteopedia's Cation pi Category]] (no hyphen) | ||
| - | *[[:Category:Cation-pi_interaction|Entries in Proteopedia's Cation-pi interaction Category]] | ||
| - | |||
| - | ==Literature References== | ||
| - | <references/> | ||
| - | |||
| - | ==Additional Literature and Resources== | ||
| - | <ref group="xtra">PMID: 8539615</ref><ref group="xtra">PMID: 12162741</ref><ref group="xtra">PMID: 15225993</ref><ref group="xtra">PMID: 15726638</ref><ref group="xtra">PMID: 15769516</ref><ref group="xtra">PMID: 17154537</ref><ref group="xtra">PMID: 15726638</ref><ref group="xtra">PMID: 17513416</ref><ref group="xtra">PMID: 18759391</ref><ref group="xtra">PMID: 1678899</ref><ref group="xtra">PMID: 15098021</ref> | ||
| - | <references group="xtra"/> | ||
| - | *Felder C, Jiang HL, Zhu WL, Chen, KX, Silman I, Botti, SA, Sussman JL. Quantum/classical mechanical comparison of cation-π interactions between tetramethylammonium and benzene. J. Phys. Chem. 2001 A 105: 1326-1333. [http://www.weizmann.ac.il/sb/faculty_pages/Sussman/papers/2001_Felder_JPC.pdf Paper.] | ||
| - | *Tan XJ, Zhu WL, Cui M, Luo XM, Gu JD, Silman I, Sussman JL, Jiang HL, Ji RY, Chen KX. Noncovalent interaction or chemical bonding between alkaline earth cations and benzene? A quantum chemistry study with MP2 and density-functional theory methods. Chem. Phys. Lett. 2001 349:113-122. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TFN-44B4HC9-3&_user=1516330&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000053443&_version=1&_urlVersion=0&_userid=1516330&md5=aafae3391c122c6fdbfdf1d5c93e76a0 Abstract] | ||
| - | *Sussman JL, Silman I. Acetylcholinesterase: structure and use as a model for specific cation-protein interactions. Curr. Opin. Struct. Biol. 1992 2:721-729. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VS6-488FR7B-18&_user=10&_handle=V-WA-A-W-WV-MsSAYZW-UUW-U-AACCVECUCE-AACBUDCYCE-WADDYDE-WV-U&_fmt=summary&_coverDate=10%2F31%2F1992&_rdoc=12&_orig=browse&_srch=%23toc%236254%231992%23999979994%23411862!&_cdi=6254&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=311482e5def4d93a8ddc6423a65772db Abstract] | ||
| - | *[http://www.umass.edu/microbio/chime/pe_beta/pe/protexpl/cationpi.htm The Introduction, Gallery & Tutorial for Cation-Pi Interactions from Eric Martz's Protein Explorer] | ||
| - | *[http://en.wikipedia.org/wiki/Cation-pi_interaction Wikipedia Cation-pi interaction page] | ||
| - | |||
| - | ==Content Attribution== | ||
| - | The initial content of this page was adapted, with the permission of [[User:Eric Martz|Eric Martz]], from the [http://proteinexplorer.org/igloss.htm Glossary] and [http://proteinexplorer.org/cationpi.htm Introduction, Gallery & Tutorial for Cation-Pi Interactions] that accompanies [[Protein Explorer]]. | ||
| - | |||
| - | [[Category:Cation_pi]] | ||
| - | [[Category:Cation-pi]] | ||
| - | [[Category:Cation-pi_interaction]] | ||
Revision as of 19:14, 19 April 2010
|
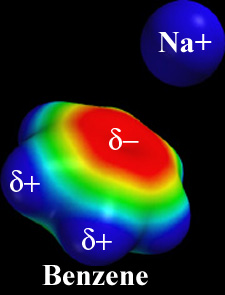
| Cation-pi orbital interaction. Surfaces colored by electrostatic potential. Image adapted from one kindly provided by Dennis Dougherty. |
Cationic moieties, such as sidechain nitrogens (lysine or arginine) or metal cations, that are within 6.0 Å of the face of an aromatic ring (phenylalanine, tyrosine, or tryptophan) may engage in polar interactions called cation-pi orbital interactions (cation-π interactions).
Butyrylcholinesterase, also known as pseudocholinesterase, BCHE or BuChE, is an enzyme that, in humans, is encoded by the BCHE gene. Butyrylcholinesterase is also called serum cholinesterase. It is very similar to the neuronal acetylcholinesterase, and is a non-specific cholinesterase found in the blood plasma, which hydrolyses many different choline esters. Butyrylcholine is a synthetic compound and does not occur in the body naturally. It is used as a tool to distinguish between acetyl- and butyrylcholinesterase. The structure for this protein is available in its unmodified form (2pm8 and 1p0i)
One of the main functions of butyrylcholinesterase is its ability to remove and clean away organophosphorus nerve agents. This function and its inhibitors has been intensibely studied by solving protein structure of BChE in combination with organophosphate inhibitors and substrate inhibitors.
Proteopedia examples on these experiments and related publications are
BChE+organophosphate inhibitors causing irreversible inhibition
