6-deoxyerythronolide B synthase (DEBS)
From Proteopedia
| Line 2: | Line 2: | ||
| - | Polyketides are a large class of natural products produced by bacteria, fungi, and plants. Polyketides exhibit diverse structures and a wide variety of biological activities, which includes antibiotic, antitumor, anticancer, and other biological properties. | + | Polyketides are a large class of natural products produced by bacteria, fungi, and plants. Polyketides exhibit diverse structures and a wide variety of biological activities, which includes antibiotic, antitumor, anticancer, and other biological properties. |
| - | In nature, polyketides are synthesized by large multifunctional enzymes called polyketide synthases (PKS). Among several characterized PKSs, the biosynthesis of the polyketide Core of erythromycin A, 6-deoxyerythronolide B (6-dEB), has provided the paradigm for understanding the structure and function of the PKSs that are responsible for assembling complex polyketides . | + | In nature, polyketides are synthesized by large multifunctional enzymes called polyketide synthases (PKS). Among several characterized PKSs, the biosynthesis of the polyketide Core of erythromycin A, 6-deoxyerythronolide B (6-dEB), has provided the paradigm for understanding the structure and function of the PKSs that are responsible for assembling complex polyketides . |
[[Image:DEBS.png|frame|6-deoxyerythronolide B synthase (DEBS)|left|300px]] | [[Image:DEBS.png|frame|6-deoxyerythronolide B synthase (DEBS)|left|300px]] | ||
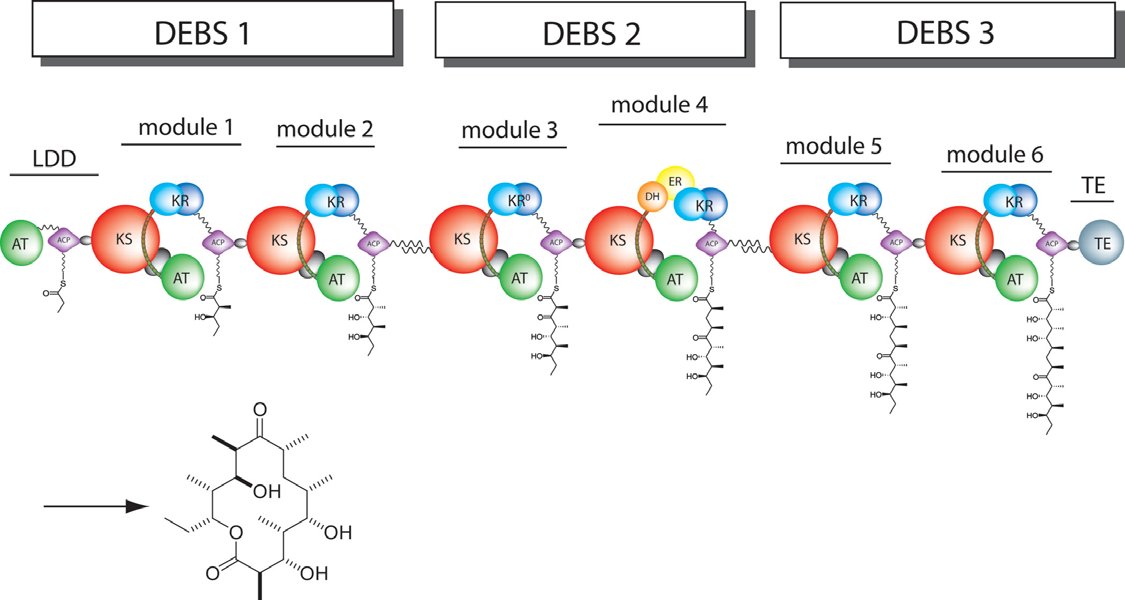
| - | The 6-deoxyerythronolide B synthase (DEBS), which catalyzes the formation of 6-dEB, consists of three large polypeptides, DEBS1, DEBS2 and DEBS3, each above 300 kD in size, and each polypeptide in DEBS is composed of two modules, where a module includes all the catalytic domains responsible for one round of chain extension and modification on the growing polyketide intermediate. Each module contains at least three essential domains; a β-keto acyl synthase or ketosynthase (KS), an acyl transferase (AT) and an acyl carrier protein (ACP). All of them catalyze extender units adding onto the growing polyketide chain. In detail, AT domain selects the appropriate carbon extender unit and transfers the units from acyl-CoA metabolite onto the phosphopantetheinyl arm of ACP, and KS domains catalyzes an ACP-bound extender unit adding onto the elongating polyketide chain from the previous module or loading module by decarboxylative condensation. | ||
| - | After the extender unit was added onto the elongating polyketide chain, it can be further processed by optional tailoring domains, including ketoreductases (KRs), dehydratases (DHs), and enoyl reductases (ERs), to change the oxidation state of each ketide-unit and to yield a hydroxyl, enoyl, or methylene group that may be present in the final product. In addition, the thioesterase (TE) domain that located at the C-terminus of module 6 of DEBS catalyzes the macrocyclization and releases the final product 6-dEB. | + | The 6-deoxyerythronolide B synthase (DEBS), which catalyzes the formation of 6-dEB, consists of three large subunits, DEBS1, DEBS2 and DEBS3, each containing two modules and above 300 kD in size. There are 2 catalytic domains in a loading module and 26 domains in six extender modules, and the loading module is responsible for the priming, and each extender module is responsible for one round of chain extension and possible modification on the elongating polyketide intermediate. Each extender module contains at least three essential domains: a β-keto acyl synthase or ketosynthase (KS), an acyl transferase (AT) and an acyl carrier protein (ACP). In detail, AT domain selects the appropriate carbon extender unit and transfers the units from acyl-CoA metabolite onto the phosphopantethinyl arm of ACP, and KS domains take over the polyketide chain from the previous module or loading module and further catalyze the chain elongation reaction by adding an ACP-bound extender unit through decarboxylative condensation. |
| + | After the extender unit was added onto the elongating polyketide chain, it can be further processed by optional tailoring domains, including ketoreductases (KRs), dehydratases (DHs), and enoyl reductases (ERs), to change the oxidation state of each ketide-unit and to yield a hydroxyl, enoyl, or methylene group that may be present in the final product. In addition, the thioesterase (TE) domain that located at the C-terminus of module 6 of DEBS catalyzes the macrocyclization and releases the final product 6-dEB. | ||
Revision as of 07:23, 4 May 2010
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Polyketides are a large class of natural products produced by bacteria, fungi, and plants. Polyketides exhibit diverse structures and a wide variety of biological activities, which includes antibiotic, antitumor, anticancer, and other biological properties.
In nature, polyketides are synthesized by large multifunctional enzymes called polyketide synthases (PKS). Among several characterized PKSs, the biosynthesis of the polyketide Core of erythromycin A, 6-deoxyerythronolide B (6-dEB), has provided the paradigm for understanding the structure and function of the PKSs that are responsible for assembling complex polyketides .
The 6-deoxyerythronolide B synthase (DEBS), which catalyzes the formation of 6-dEB, consists of three large subunits, DEBS1, DEBS2 and DEBS3, each containing two modules and above 300 kD in size. There are 2 catalytic domains in a loading module and 26 domains in six extender modules, and the loading module is responsible for the priming, and each extender module is responsible for one round of chain extension and possible modification on the elongating polyketide intermediate. Each extender module contains at least three essential domains: a β-keto acyl synthase or ketosynthase (KS), an acyl transferase (AT) and an acyl carrier protein (ACP). In detail, AT domain selects the appropriate carbon extender unit and transfers the units from acyl-CoA metabolite onto the phosphopantethinyl arm of ACP, and KS domains take over the polyketide chain from the previous module or loading module and further catalyze the chain elongation reaction by adding an ACP-bound extender unit through decarboxylative condensation. After the extender unit was added onto the elongating polyketide chain, it can be further processed by optional tailoring domains, including ketoreductases (KRs), dehydratases (DHs), and enoyl reductases (ERs), to change the oxidation state of each ketide-unit and to yield a hydroxyl, enoyl, or methylene group that may be present in the final product. In addition, the thioesterase (TE) domain that located at the C-terminus of module 6 of DEBS catalyzes the macrocyclization and releases the final product 6-dEB.
Contents |
KS-AT
Molecular Playground banner: KS-AT is responsible for one round of chain extension, "the carbon adding worker".
|
ACP
Molecular Playground banner: carrying the carbon extender unit, "the communicator".
|
DH
Molecular Playground banner: make modification on beta-keto-acyl-ACP, "the decorator".
|
TE
Molecular Playground banner: cyclize the molecule, "the closer".
|
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Tsung-Yi Lin, Alexander Berchansky, Lawrence Sheringham Borketey, Joel L. Sussman, Jon Amoroso, David Canner, Jaime Prilusky