User:Mary Ball/AFP
From Proteopedia
(New page: {{Seed}} 200px <!-- The line below this paragraph, containing "STRUCTURE_1wfb", creates the "Structure Box" on the page. You may change the PDB parameter (which se...) |
|||
| Line 10: | Line 10: | ||
{{STRUCTURE_1wfb| PDB=1wfb | SCENE= }} | {{STRUCTURE_1wfb| PDB=1wfb | SCENE= }} | ||
| - | ===WINTER FLOUNDER ANTIFREEZE PROTEIN | + | ===WINTER FLOUNDER ANTIFREEZE PROTEIN=== |
| + | {{Abstract | ||
| + | |ABSTRACT= | ||
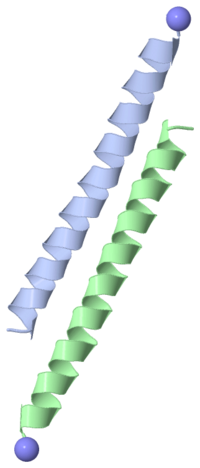
| + | Type I antifreeze protein (AFP) from winter flounder is an alanine-rich, 37 amino acid, single alpha-helix that contains three 11 amino acid repeats (Thr-X(2)-Asx-X(7)), where X is generally Ala. The regularly spaced Thr, Asx and Leu residues lie on one face of the helix and have traditionally been thought to form hydrogen bonds and van der Waals interactions with the ice surface. Recently, substitution experiments have called into question the importance of Leu and Asn for ice-binding. Sequence alignments of five type I AFP isoforms show that Leu and Asn are not well conserved, whereas Ala residues adjacent to the Thr, at right angles to the Leu/Asn-rich face, are completely conserved. To investigate the role of these Ala residues, a series of Ala to Leu steric mutations was made at various points around the helix. All the substituted peptides were fully alpha-helical and remained as monomers in solution. Wild-type activity was retained in A19L and A20L. A17L, where the substitution lies adjacent to the Thr-rich face, had no detectable antifreeze activity. The nearby A21L substitution had 10% wild-type activity and demonstrated weak interactions with the ice surface. We propose a new ice-binding face for type I AFP that encompasses the conserved Ala-rich surface and adjacent Thr. | ||
| + | |REFERENCE=New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10601644 10601644] | ||
| + | }} | ||
<!-- | <!-- | ||
| - | The line below this paragraph, {{ | + | The line below this paragraph, {{ABSTRACT_PUBMED_10601644}}, adds the Publication Abstract to the page |
| - | (as it appears on PubMed at http://www.pubmed.gov), where | + | (as it appears on PubMed at http://www.pubmed.gov), where 10601644 is the PubMed ID number. |
--> | --> | ||
| - | + | ||
==About this Structure== | ==About this Structure== | ||
| Line 34: | Line 39: | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Jan 21 08:56:47 2010'' | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Jan 21 08:56:47 2010'' | ||
| + | |||
| + | ===WINTER FLOUNDER ANTIFREEZE PROTEIN ISOFORM HPLC6 AT-180 DEGREES C=== | ||
| + | |||
| + | |||
| + | <!-- | ||
| + | The line below this paragraph, {{ABSTRACT_PUBMED_7760940}}, adds the Publication Abstract to the page | ||
| + | (as it appears on PubMed at http://www.pubmed.gov), where 7760940 is the PubMed ID number. | ||
| + | --> | ||
| + | {{ABSTRACT_PUBMED_7760940}} | ||
Revision as of 11:48, 9 June 2010
| |||||||||
| 1wfb, resolution 1.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||||
| Related: | 1wfa | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
WINTER FLOUNDER ANTIFREEZE PROTEIN
Type I antifreeze protein (AFP) from winter flounder is an alanine-rich, 37 amino acid, single alpha-helix that contains three 11 amino acid repeats (Thr-X(2)-Asx-X(7)), where X is generally Ala. The regularly spaced Thr, Asx and Leu residues lie on one face of the helix and have traditionally been thought to form hydrogen bonds and van der Waals interactions with the ice surface. Recently, substitution experiments have called into question the importance of Leu and Asn for ice-binding. Sequence alignments of five type I AFP isoforms show that Leu and Asn are not well conserved, whereas Ala residues adjacent to the Thr, at right angles to the Leu/Asn-rich face, are completely conserved. To investigate the role of these Ala residues, a series of Ala to Leu steric mutations was made at various points around the helix. All the substituted peptides were fully alpha-helical and remained as monomers in solution. Wild-type activity was retained in A19L and A20L. A17L, where the substitution lies adjacent to the Thr-rich face, had no detectable antifreeze activity. The nearby A21L substitution had 10% wild-type activity and demonstrated weak interactions with the ice surface. We propose a new ice-binding face for type I AFP that encompasses the conserved Ala-rich surface and adjacent Thr.
New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:10601644
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1WFB is a 2 chains structure with sequences from Pseudopleuronectes americanus. The December 2009 RCSB PDB Molecule of the Month feature on Antifreeze Proteins by David Goodsell is 10.2210/rcsb_pdb/mom_2009_12. Full crystallographic information is available from OCA.
Reference
- Sicheri F, Yang DS. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940 doi:http://dx.doi.org/10.1038/375427a0
Page seeded by OCA on Thu Jan 21 08:56:47 2010
WINTER FLOUNDER ANTIFREEZE PROTEIN ISOFORM HPLC6 AT-180 DEGREES C
Antifreeze proteins provide fish with protection against the freezing effect of polar environments by binding to ice surfaces and inhibiting growth of ice crystals. We present the X-ray crystal structure at 1.5 A resolution of a lone alpha-helical antifreeze protein from winter flounder, which provides a detailed look at its ice-binding features. These consist of four repeated ice-binding motifs, the side chains of which are inherently rigid or restrained by pair-wise side-chain interactions to form a flat binding surface. Elaborate amino- and carboxy-terminal cap structures are also present, which explain the protein's rich alpha-helical content in solution. We propose an ice-binding model that accounts for the binding specificity of the antifreeze protein along the <0112> axes of the (2021) ice planes.
Ice-binding structure and mechanism of an antifreeze protein from winter flounder., Sicheri F, Yang DS, Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.