User:Mary Ball/AFP
From Proteopedia
(→WINTER FLOUNDER ANTIFREEZE PROTEIN) |
|||
| Line 13: | Line 13: | ||
{{Abstract | {{Abstract | ||
|ABSTRACT= | |ABSTRACT= | ||
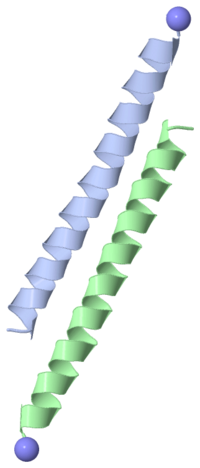
| - | Type I antifreeze protein (AFP) from winter flounder | + | Type I antifreeze protein (AFP)are grouped according to similar structure. The one shown is from the winter flounder. |
| + | This AFP consists of a chain of 37 amino acids that forms a single alpha-helix. Four threonines are evenly spaced in the chain, such that there are three 11-amino-acid repeats. In the single alpha-helix that forms, the threonines all lie on one "face" of the helix. | ||
| + | |||
| + | <scene name='User:Mary_Ball/Sandbox/Wfb/1'>WFB</scene> | ||
<scene name='User:Mary_Ball/Sandbox/Amino_acids/1'>Click here to see a labelled Ala.</scene> <scene name='User:Mary_Ball/AFP/Threonine_residues/2'>Scene with Threonine Residues Labelled</scene> | <scene name='User:Mary_Ball/Sandbox/Amino_acids/1'>Click here to see a labelled Ala.</scene> <scene name='User:Mary_Ball/AFP/Threonine_residues/2'>Scene with Threonine Residues Labelled</scene> | ||
| - | + | ||
| + | Baardsnes, et al (1999) created mutations that reduced the protein's ice-binding ability. They also compared the sequences of five different type I AFP molecules. They found that Ala residues adjacent to the Thr were completely conserved, and concluded that the repeated Threonines, along with many of the Alanines, create one "face" of the helix adapted to bind ice crystals. | ||
| + | |||
| + | |||
|REFERENCE=New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10601644 10601644] | |REFERENCE=New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10601644 10601644] | ||
| Line 24: | Line 30: | ||
(as it appears on PubMed at http://www.pubmed.gov), where 10601644 is the PubMed ID number. | (as it appears on PubMed at http://www.pubmed.gov), where 10601644 is the PubMed ID number. | ||
--> | --> | ||
| - | |||
==ABOUT THIS STRUCTURE== | ==ABOUT THIS STRUCTURE== | ||
Revision as of 14:39, 11 June 2010
| |||||||||
| 1wfb, resolution 1.50Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Standard Residues: | |||||||||
| Related: | 1wfa | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
WINTER FLOUNDER ANTIFREEZE PROTEIN
Type I antifreeze protein (AFP)are grouped according to similar structure. The one shown is from the winter flounder. This AFP consists of a chain of 37 amino acids that forms a single alpha-helix. Four threonines are evenly spaced in the chain, such that there are three 11-amino-acid repeats. In the single alpha-helix that forms, the threonines all lie on one "face" of the helix.
Baardsnes, et al (1999) created mutations that reduced the protein's ice-binding ability. They also compared the sequences of five different type I AFP molecules. They found that Ala residues adjacent to the Thr were completely conserved, and concluded that the repeated Threonines, along with many of the Alanines, create one "face" of the helix adapted to bind ice crystals.
New ice-binding face for type I antifreeze protein., Baardsnes J, Kondejewski LH, Hodges RS, Chao H, Kay C, Davies PL, FEBS Lett. 1999 Dec 10;463(1-2):87-91. PMID:10601644
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
ABOUT THIS STRUCTURE
1WFB is a 2 chains structure with sequences from Pseudopleuronectes americanus. The December 2009 RCSB PDB Molecule of the Month feature on Antifreeze Proteins by David Goodsell is 10.2210/rcsb_pdb/mom_2009_12. Full crystallographic information is available from OCA.
REFERENCE
- Sicheri F, Yang DS. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940 doi:http://dx.doi.org/10.1038/375427a0
Page seeded by OCA on Thu Jan 21 08:56:47 2010
WINTER FLOUNDER ANTIFREEZE PROTEIN ISOFORM HPLC6 AT-180 DEGREES C
Antifreeze proteins provide fish with protection against the freezing effect of polar environments by binding to ice surfaces and inhibiting growth of ice crystals. We present the X-ray crystal structure at 1.5 A resolution of a lone alpha-helical antifreeze protein from winter flounder, which provides a detailed look at its ice-binding features. These consist of four repeated ice-binding motifs, the side chains of which are inherently rigid or restrained by pair-wise side-chain interactions to form a flat binding surface. Elaborate amino- and carboxy-terminal cap structures are also present, which explain the protein's rich alpha-helical content in solution. We propose an ice-binding model that accounts for the binding specificity of the antifreeze protein along the <0112> axes of the (2021) ice planes.
Ice-binding structure and mechanism of an antifreeze protein from winter flounder., Sicheri F, Yang DS, Nature. 1995 Jun 1;375(6530):427-31. PMID:7760940
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.