1ax8
From Proteopedia
| Line 20: | Line 20: | ||
==About this Structure== | ==About this Structure== | ||
| - | 1AX8 is a 1 chain structure | + | 1AX8 is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1AX8 OCA]. |
==Reference== | ==Reference== | ||
| Line 31: | Line 31: | ||
[[Category: Wery, J P.]] | [[Category: Wery, J P.]] | ||
[[Category: Zhang, F.]] | [[Category: Zhang, F.]] | ||
| + | [[Category: Cytokine]] | ||
[[Category: Diabetes]] | [[Category: Diabetes]] | ||
[[Category: Helical cytokine]] | [[Category: Helical cytokine]] | ||
| Line 36: | Line 37: | ||
[[Category: Obesity]] | [[Category: Obesity]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Wed Jul 14 15:54:45 2010'' |
Revision as of 11:48, 14 July 2010
| |||||||||
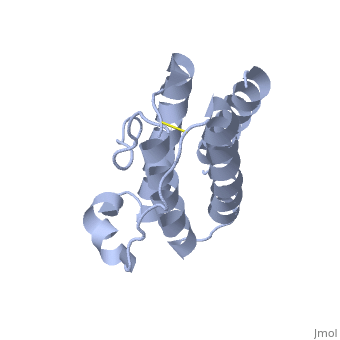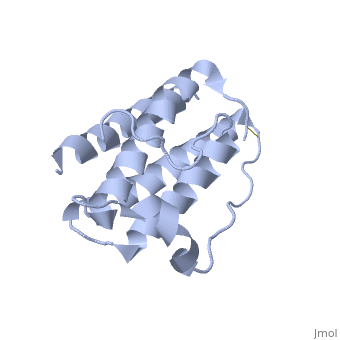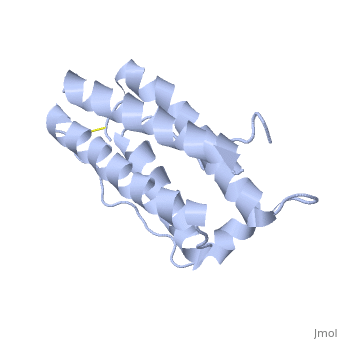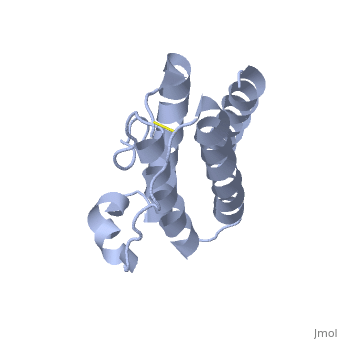
| 1ax8, resolution 2.40Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene: | OBESE GENE (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
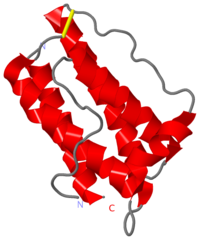
HUMAN OBESITY PROTEIN, LEPTIN
Mutations in the obese gene (OB) or in the gene encoding the OB receptor(OB-R) result in obesity, infertility and diabetes in a variety of mouse phenotypes. The demonstration that OB protein (also known as leptin) can normalize body weight in ob/ob mice has generated enormous interest. Most human obesity does not appear to result from a mutant form of leptin: rather, serum leptin concentrations are increased and there is an apparent inability to transport it to the central nervous system (CNS). Injection of leptin into the CNS of overfed rodents resistant to peripheral administration was found to induce biological activity. Consequently, for the leptin to act as a weight-lowering hormone in human obesity, it appears that appropriate concentrations must be present in the CNS. This places a premium on understanding the structure of the hormone in order to design more potent and selective agonists. Here we report the crystal structure at 2.4A resolution of a human mutant OB protein (leptin-E100) that has comparable biological activity to wild type but which crystallizes more readily. The structure reveals a four-helix bundle similar to that of the long-chain helical cytokine family.
Crystal structure of the obese protein leptin-E100., Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW, Nature. 1997 May 8;387(6629):206-9. PMID:9144295
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
1AX8 is a 1 chain structure with sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
- Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature. 1997 May 8;387(6629):206-9. PMID:9144295 doi:10.1038/387206a0
Page seeded by OCA on Wed Jul 14 15:54:45 2010