Journal:JBIC:1
From Proteopedia

(New page: This is sample page for a Journal.) |
|||
| Line 1: | Line 1: | ||
| - | This is | + | [[Image: 3dzy2.png|420px|left|thumb| Human PPARγ bound to RXRα and PPRE DNA strand, [[3dzy]]]] |
| + | {{STRUCTURE_3dzy| right| PDB=3dzy | SCENE=Peroxisome_Proliferator-Activated_Receptors/Ppar_opening4/1 |CAPTION= Crystal Structure of Human PPARγ, [[3dzy]] }} | ||
| + | The [[Peroxisome Proliferator-Activated Receptors]] (PPAR) α, δ, and γ are members of the nuclear receptor family. Since their discovery in the early 90s, it has become clear that the PPARs are essential modulators of environmental and dietary stimuli, acting as transcription factors to regulate mammalian metabolism, cellular differentiation, and tumorigenesis. The PPARs are the targets of numerous pharmaceutical drugs aimed at treating [http://en.wikipedia.org/wiki/Hyperlipidemia hypolipidemia] and [http://en.wikipedia.org/wiki/Diabetes diabetes] among other diseases.<ref>PMID:15860251</ref> | ||
| + | <br /> | ||
| + | |||
| + | |||
| + | {{TOC limit|limit=2}} | ||
| + | <br /> | ||
| + | |||
| + | ==Biological Role== | ||
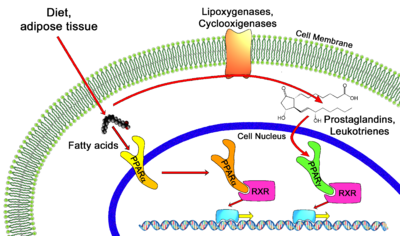
| + | [[Image: PPAR_Mechanism.png|400px|left|thumb| PPAR Mechanism of Action in the Human Body]] | ||
| + | Transcription of individual genes in eukaryotic cells is controlled very precisely at a number of different levels. One key level is the binding of specific [[DNA]] binding transcriptional factors such as nuclear receptors, to facilitate RNA polymerase function. Unliganded PPARs form a heterodimer with retinoid X receptor (RXR), specifically RXRα. This heterodimer binds to the Peroxisome Proliferator Response Element (PPRE), a specific DNA sequence present in the promoter region of PPAR-regulated genes. <ref>PMID:11330046</ref> Also associated with this unliganded heterodimer is a co-repressor complex which possesses histone deacetylation activity, enforcing a tight chromatin structure which prevents gene transcription. <ref>PMID:15681609</ref> This co-repressor complex is released upon ligand binding (typical ligands include lipids and eicosanoids), allowing various co-activators and co-activator-associated proteins to be recruited. These protein complexes modulate chromatin remodeling as well as facilitate DNA unwinding and linkage to RNA polymerase II machinery, to begin transcription. Some PPAR related co-activators include CBP (Histone Acetylation), SRC-1,2,3 (Chromatin Acetylation), <ref>pmid:7539101</ref> PGC-1 (Recruit [http://en.wikipedia.org/wiki/Histone_acetyltransferase HAT activities]), PRIC-285,320 (Chromatin Remodeling via Helicase activity)<ref>PMID:11158331</ref>and PIMT (RNA Capping via methyltransferase activity)<ref>PMID:10381882</ref>. | ||
Revision as of 13:32, 22 August 2010
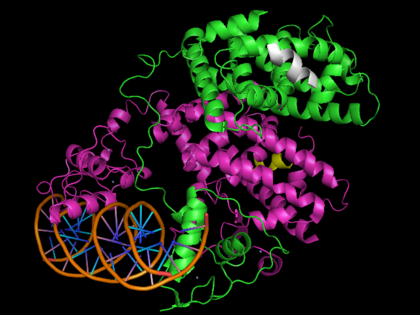
Template:STRUCTURE 3dzy
The Peroxisome Proliferator-Activated Receptors (PPAR) α, δ, and γ are members of the nuclear receptor family. Since their discovery in the early 90s, it has become clear that the PPARs are essential modulators of environmental and dietary stimuli, acting as transcription factors to regulate mammalian metabolism, cellular differentiation, and tumorigenesis. The PPARs are the targets of numerous pharmaceutical drugs aimed at treating hypolipidemia and diabetes among other diseases.[1]
Contents |
Biological Role
Transcription of individual genes in eukaryotic cells is controlled very precisely at a number of different levels. One key level is the binding of specific DNA binding transcriptional factors such as nuclear receptors, to facilitate RNA polymerase function. Unliganded PPARs form a heterodimer with retinoid X receptor (RXR), specifically RXRα. This heterodimer binds to the Peroxisome Proliferator Response Element (PPRE), a specific DNA sequence present in the promoter region of PPAR-regulated genes. [2] Also associated with this unliganded heterodimer is a co-repressor complex which possesses histone deacetylation activity, enforcing a tight chromatin structure which prevents gene transcription. [3] This co-repressor complex is released upon ligand binding (typical ligands include lipids and eicosanoids), allowing various co-activators and co-activator-associated proteins to be recruited. These protein complexes modulate chromatin remodeling as well as facilitate DNA unwinding and linkage to RNA polymerase II machinery, to begin transcription. Some PPAR related co-activators include CBP (Histone Acetylation), SRC-1,2,3 (Chromatin Acetylation), [4] PGC-1 (Recruit HAT activities), PRIC-285,320 (Chromatin Remodeling via Helicase activity)[5]and PIMT (RNA Capping via methyltransferase activity)[6].
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Eran Hodis, Alexander Berchansky, Jaime Prilusky, Joel L. Sussman