We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:JBIC:1
From Proteopedia
(Difference between revisions)

| Line 1: | Line 1: | ||
| + | Crystal structure of the zinc, cobalt and iron containing adenylate kinase from Desulfov ibrio gigas: a novel metal containing adenylate kinase from Gram-negative bacteria <br /> | ||
| + | By Dr. Mukhopadhyay & Abhik Mukhopadhyay <br /> | ||
| + | Journal of Biological Inorganic Chemistry - JBIC-10-04-00074.R2 <br /> | ||
| + | |||
[[Image: 3dzy2.png|420px|left|thumb| Human PPARγ bound to RXRα and PPRE DNA strand, [[3dzy]]]] | [[Image: 3dzy2.png|420px|left|thumb| Human PPARγ bound to RXRα and PPRE DNA strand, [[3dzy]]]] | ||
| - | {{STRUCTURE_3dzy| right| PDB=3dzy | SCENE=Peroxisome_Proliferator-Activated_Receptors/Ppar_opening4/1 |CAPTION= Crystal Structure of Human PPARγ, [[3dzy]] }} | ||
| - | The [[Peroxisome Proliferator-Activated Receptors]] (PPAR) α, δ, and γ are members of the nuclear receptor family. Since their discovery in the early 90s, it has become clear that the PPARs are essential modulators of environmental and dietary stimuli, acting as transcription factors to regulate mammalian metabolism, cellular differentiation, and tumorigenesis. The PPARs are the targets of numerous pharmaceutical drugs aimed at treating [http://en.wikipedia.org/wiki/Hyperlipidemia hypolipidemia] and [http://en.wikipedia.org/wiki/Diabetes diabetes] among other diseases.<ref>PMID:15860251</ref> | ||
| - | <br /> | ||
| - | |||
| - | |||
| - | {{TOC limit|limit=2}} | ||
| - | <br /> | ||
| - | = | + | <scene name='Journal:JBIC:1/Cobalt_bound/1'>TextToBeDisplayed</scene> |
| - | + | ||
| - | + | ||
Revision as of 15:11, 22 August 2010
Crystal structure of the zinc, cobalt and iron containing adenylate kinase from Desulfov ibrio gigas: a novel metal containing adenylate kinase from Gram-negative bacteria
By Dr. Mukhopadhyay & Abhik Mukhopadhyay
Journal of Biological Inorganic Chemistry - JBIC-10-04-00074.R2
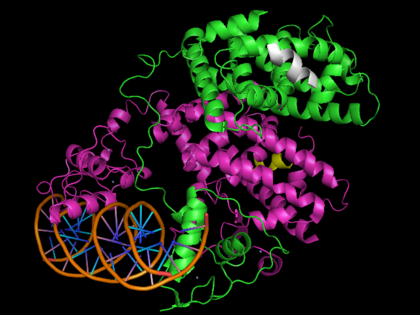
Human PPARγ bound to RXRα and PPRE DNA strand, 3dzy
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Eran Hodis, Alexander Berchansky, Jaime Prilusky, Joel L. Sussman
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.
