Acetylcholinesterase
From Proteopedia
| Line 26: | Line 26: | ||
Thus drugs that are mild inhibitors of AChE, like Tacrine, E2020 (Aricept) and the natural Chinese produce Huperzine appear to retard symptoms of AD. | Thus drugs that are mild inhibitors of AChE, like Tacrine, E2020 (Aricept) and the natural Chinese produce Huperzine appear to retard symptoms of AD. | ||
| - | <applet load='1ea5_rot.pdb' size='300' color='white' frame='true' spin='on' caption='AChE' align='right' script='Acetylcholinesterase/ | + | <applet load='1ea5_rot.pdb' size='300' color='white' frame='true' spin='on' caption='AChE' align='right' script='Acetylcholinesterase/Active_site_gorge/4' |
| - | + | ||
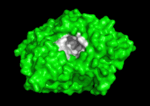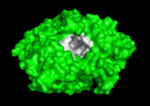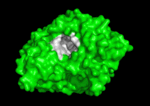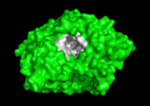
The active site region of this enzyme has two sites, a catalytic site and a peripheral site, which helps prebind the substrate and direct it toward the active site. When the 3-D structure was first determined, the big surprise was that the active site was deep inside the protein, at the end or base of a <scene name='Acetylcholinesterase/Ache_gorge/1'>deep and narrow gorge </scene>, lined with aromatic residues, with the peripheral site at the top or lip of this gorge. Amazingly, there were no acidic or negatively charged | The active site region of this enzyme has two sites, a catalytic site and a peripheral site, which helps prebind the substrate and direct it toward the active site. When the 3-D structure was first determined, the big surprise was that the active site was deep inside the protein, at the end or base of a <scene name='Acetylcholinesterase/Ache_gorge/1'>deep and narrow gorge </scene>, lined with aromatic residues, with the peripheral site at the top or lip of this gorge. Amazingly, there were no acidic or negatively charged | ||
residues anywhere in these 2 sites or along this gorge, as would be expected to | residues anywhere in these 2 sites or along this gorge, as would be expected to | ||
Revision as of 12:18, 22 January 2008
|
Key Enzyme in the Nervous System
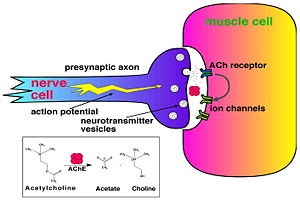
Acetylcholinesterase (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, acetylcholine (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors.
Solution of the 3D structure of Torpedo californica AChE (TcAChE) in 1991 (Sussman et al. & Silman (1991)) opened up new horizons in research on an enzyme that had already been the subject of intensive investigation. The unanticipated structure of this extremely rapid enzyme, in which the active site was found to be buried at the bottom of a , lined by aromatic residues, led to a revision of the views then held concerning substrate traffic, recognition, and hydrolysis (Botti et al. Sussman & Silman (1999)). This led to a series of theoretical and experimental studies, which took advantage of recent advances in theoretical techniques for treatment of proteins, such as molecular dynamics and electrostatics, and of sitedirected mutagenesis, utilizing suitable expression systems.
Alzheimer’s disease (AD) is a debilitating brain disease that occurs in around 10% of the elderly and, as yet, there is no known cure. At present, the most widely used treatments consist are medications that attempt to increase the brain’s levels of ACh, whose levels decrease with onset of disease. These drugs work by interfering with AChE. Thus drugs that are mild inhibitors of AChE, like Tacrine, E2020 (Aricept) and the natural Chinese produce Huperzine appear to retard symptoms of AD.
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu