User:Enrico Ravera/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
Maximum Occurrence (MO) refers to a method for making rigorous numerical assessments on the conformational space of flexible biological macromolecules<ref>doi:10.1021/ja1063923</ref>. | Maximum Occurrence (MO) refers to a method for making rigorous numerical assessments on the conformational space of flexible biological macromolecules<ref>doi:10.1021/ja1063923</ref>. | ||
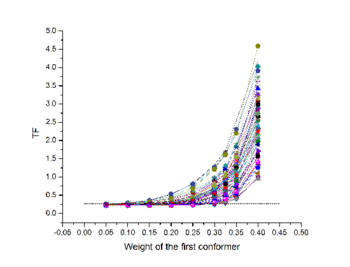
| - | The Maximum Occurrence is the maximum percent of time that a macromolecule can spend in a given conformation and still be compatible with the experimental data (see Figure). | + | The Maximum Occurrence is the maximum percent of time that a macromolecule can spend in a given conformation and still be compatible with the experimental data (see Figure 1). |
| - | [[Image:MO_profiles.png|thumbnail|350px|Maximum Occurrence profiles for some conformers of Calcium-loaded calmodulin. Dashed line indicates the maximum disagreement within experimental and calculated data.]] | + | [[Image:MO_profiles.png|thumbnail|350px|Figure 1: Maximum Occurrence profiles for some conformers of Calcium-loaded calmodulin. Dashed line indicates the maximum disagreement within experimental and calculated data.]] |
== Case Study: Calmodulin == | == Case Study: Calmodulin == | ||
Revision as of 12:37, 23 September 2010
Maximum Occurrence (MO) refers to a method for making rigorous numerical assessments on the conformational space of flexible biological macromolecules[1]. The Maximum Occurrence is the maximum percent of time that a macromolecule can spend in a given conformation and still be compatible with the experimental data (see Figure 1).
Case Study: Calmodulin
Maximum Occurrence profiles were calculated for N60D Calmodulin[2](PDB ENTRIES 1sw8,2k0j,2k61). Calmodulin is a two-domain protein experiencing high mobility in the central region[3][4]. Paramagnetic NMR restraints as pseudocontact shift (PCS) and self-orientation residual dipolar couplings (RDC) provided further insight in the description of such conformational heterogeneity[5]. (PDB ENTRIES 1sw8,2k0j,2k61)
Bibliography
- ↑ Bertini I, Giachetti A, Luchinat C, Parigi G, Petoukhov MV, Pierattelli R, Ravera E, Svergun DI. Conformational Space of Flexible Biological Macromolecules from Average Data. J Am Chem Soc. 2010 Sep 7. PMID:20822180 doi:10.1021/ja1063923
- ↑ Bertini I, Gelis I, Katsaros N, Luchinat C, Provenzani A. Tuning the affinity for lanthanides of calcium binding proteins. Biochemistry. 2003 Jul 8;42(26):8011-21. PMID:12834353 doi:10.1021/bi034494z
- ↑ doi: https://dx.doi.org/10.1021/bi00138a005
- ↑ doi: https://dx.doi.org/10.1021/bi00403a011
- ↑ Bertini I, Del Bianco C, Gelis I, Katsaros N, Luchinat C, Parigi G, Peana M, Provenzani A, Zoroddu MA. Experimentally exploring the conformational space sampled by domain reorientation in calmodulin. Proc Natl Acad Sci U S A. 2004 May 4;101(18):6841-6. Epub 2004 Apr 20. PMID:15100408 doi:10.1073/pnas.0308641101