Molecular Playground/YKL-40
From Proteopedia
| Line 3: | Line 3: | ||
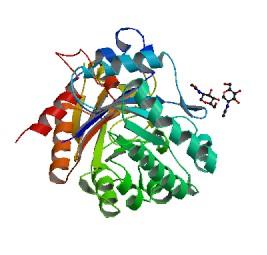
[[Image:YKL-40.jpg|frame|Crystal Structure of YKL-40]] | [[Image:YKL-40.jpg|frame|Crystal Structure of YKL-40]] | ||
| - | + | ==YKL-40, a Secreted Glycoprotein== | |
YKL-40 is named for its first three amino acid residues--tyrosine, lysine, and leucine-- and its molecular weight, 40 kDa. It is a member of the chitinase family of proteins, although it has no chitinase activity. It has been implicated in the progession of inflammatory diseases, diabetes, and many cancers. It is one of the most up-regulated genes in glioblastoma multiform (GBM), the most common malignacy of the brain and central nervous system. High YKL-40 serum levels correlate with the worst prognosis for many cancers, especially in GBM. It is thought to increase tumor angiogenesis and endothelial cell migration into tumors. | YKL-40 is named for its first three amino acid residues--tyrosine, lysine, and leucine-- and its molecular weight, 40 kDa. It is a member of the chitinase family of proteins, although it has no chitinase activity. It has been implicated in the progession of inflammatory diseases, diabetes, and many cancers. It is one of the most up-regulated genes in glioblastoma multiform (GBM), the most common malignacy of the brain and central nervous system. High YKL-40 serum levels correlate with the worst prognosis for many cancers, especially in GBM. It is thought to increase tumor angiogenesis and endothelial cell migration into tumors. | ||
| Line 13: | Line 13: | ||
<scene name='Ralph_A._Francescone_III/sandbox_1/Ligand_bound_state/1'>Ligand Bound State</scene> | <scene name='Ralph_A._Francescone_III/sandbox_1/Ligand_bound_state/1'>Ligand Bound State</scene> | ||
| + | ==Additional Resources== | ||
| + | For additional information, see: [[Diabetes & Hypoglycemia]] | ||
| + | <br /> | ||
| - | + | ==References== | |
1.) Houston, D.R., Recklies, A.D., Krupa, J.C.,Van Aalten, D.M.F. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes.(2003) J.Biol.Chem. 278: 30206. | 1.) Houston, D.R., Recklies, A.D., Krupa, J.C.,Van Aalten, D.M.F. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes.(2003) J.Biol.Chem. 278: 30206. | ||
Revision as of 15:11, 1 October 2010
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
YKL-40, a Secreted Glycoprotein
YKL-40 is named for its first three amino acid residues--tyrosine, lysine, and leucine-- and its molecular weight, 40 kDa. It is a member of the chitinase family of proteins, although it has no chitinase activity. It has been implicated in the progession of inflammatory diseases, diabetes, and many cancers. It is one of the most up-regulated genes in glioblastoma multiform (GBM), the most common malignacy of the brain and central nervous system. High YKL-40 serum levels correlate with the worst prognosis for many cancers, especially in GBM. It is thought to increase tumor angiogenesis and endothelial cell migration into tumors.
| |||||||
| 1hjx, resolution 1.85Å () | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||
| Related: | 1hjv, 1hjw, 1la7 | ||||||
| |||||||
| |||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Click here for a stick representation of the ligands bound to YKL-40 (Glycerol, N-ACETYL-D-GLUCOSAMINE, SULFATE ION):
Additional Resources
For additional information, see: Diabetes & Hypoglycemia
References
1.) Houston, D.R., Recklies, A.D., Krupa, J.C.,Van Aalten, D.M.F. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes.(2003) J.Biol.Chem. 278: 30206.
2.) Shao et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene (2009) 28, 4456–4468.
Molecular Playground banner: YKL-40, A New Cancer Biomarker
Proteopedia Page Contributors and Editors (what is this?)
Ralph A. Francescone III, Michal Harel, David Canner, Lynmarie K Thompson