Interleukin-10
From Proteopedia
m (Sandbox 165 moved to Interleukin-10) |
|||
| Line 1: | Line 1: | ||
| - | == '''Interleukin-10''' == | ||
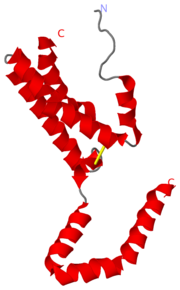
[[Image:2h24.png|right|thumb| Figure 1. IL-10 N-Terminus of helix A ]] | [[Image:2h24.png|right|thumb| Figure 1. IL-10 N-Terminus of helix A ]] | ||
'''[[2H24]]''' is the PDB id assigned to Interleukin-10 (IL-10) after the determination of the crystal structure. IL-10 is a ternary complex that requires specific assembly for proper function. The IL-10 complex is composed of IL-10,IL-10R1,IL-10R2 (R=receptor). The initial step is the formation of IL-10 and IL-10R1, a binary complex that generates a conformational change. This conformational change is required for IL-10R2 to be able to associate with the binary complex and form the ternary complex <ref name="ref1">PMID:16982608</ref>. | '''[[2H24]]''' is the PDB id assigned to Interleukin-10 (IL-10) after the determination of the crystal structure. IL-10 is a ternary complex that requires specific assembly for proper function. The IL-10 complex is composed of IL-10,IL-10R1,IL-10R2 (R=receptor). The initial step is the formation of IL-10 and IL-10R1, a binary complex that generates a conformational change. This conformational change is required for IL-10R2 to be able to associate with the binary complex and form the ternary complex <ref name="ref1">PMID:16982608</ref>. | ||
Revision as of 13:41, 13 October 2010
2H24 is the PDB id assigned to Interleukin-10 (IL-10) after the determination of the crystal structure. IL-10 is a ternary complex that requires specific assembly for proper function. The IL-10 complex is composed of IL-10,IL-10R1,IL-10R2 (R=receptor). The initial step is the formation of IL-10 and IL-10R1, a binary complex that generates a conformational change. This conformational change is required for IL-10R2 to be able to associate with the binary complex and form the ternary complex [1].
The affinity of IL-10R1 and Il-10R2 are not considered to be dependent on the amino acid sequence but something more complex. There are several homolog IL-10s and mimic IL-10s that are able to bind and signal through the IL-10 receptor complex causing overlaying signals with cIL-10 [1]. The completed ternary complex activates the JAK/STAT signaling pathway. The completion of the ternary complex is dependent on the conformational changes that occur in the N terminus of the helix labelled A. The conformational changes occur when the cIL-10 binds with IL-10R1. There is a positional change of about 1 angstrom at residues between Cys-12 and Leu-46. There are structural features that allow for the large conformational changes, the structural features are a Cys-12 to Cys-108 disulfide bond at the N terminus and Leu-Leu-Leu motifat the C terminus of the AB loop[1].
IL-10
Interleukin-10 is in the class cytokine.[1] Interleukin-10 is a very powerful anti-inflammatory cytokine that is most commonly found to be produce by moncytes [2]. IL-10 has the ability to be a immunosupressive as well as a anti-angiogenic this means that it has the ability both promot and inhibit tumors[3]. There are many types of cytokines that have many funtions such as antiinflammatory cytokines,cytokine synthesis inhibitory factor and proinflammitory cytokines[4].
There are many interleukins that fall within the cytokine class such as IL-1,IL-2,IL-3,IL-4...IL-31,IL-32,IL-33,IL-3 and IL-35.
| |||||||||
| 2h24, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Related: | 1inr | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
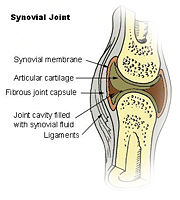
Interleukin-10 and Rheumatoid Arthritis
[5]IL-10 has been investigated for its role in patients with Rheumatoid Arthritis (RA) as well as those with Osteoarthritis (OA)[4]. RA is an autoimmune disorder that affects the synovial tissues via chronic synovitis. Chronic synovitis often results in joint destruction due to re-absorption of bone and the distruction of cartilage[6] [4]. IL-10 is found to be spontaneously produce in synovial tissue of patients with RA and OA but not in normal synovial tissue. The variation in secretion of IL-10 is thought to be 75% under the control of the genetics of the individual[6]. When IL-10 is present there is an inhibition of the proinflammatory cytokines, namely TFN-α, IL-1α, IL-1β, IL-6, IL-8 [7].
| Please do NOT make changes to this Sandbox until after April 23, 2010. Sandboxes 151-200 are reserved until then for use by the Chemistry 307 class at UNBC taught by Prof. Andrea Gorrell. |
Proteopedia Page Contributors and Editors (what is this?)
Shelly Huebert, Andrea Gorrell, Alexander Berchansky, David Canner, Jaime Prilusky, Michal Harel