We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 45
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <applet load='3LJJ' size='300' frame='true' align='right' caption='Bovine Trypsin' /> | ||
= Structure of Trypsin = | = Structure of Trypsin = | ||
| Line 22: | Line 21: | ||
<scene name='Sandbox_45/Helixhold_vanderwaals/1'>van der Waals forces</scene> | <scene name='Sandbox_45/Helixhold_vanderwaals/1'>van der Waals forces</scene> | ||
| + | <applet load='3LJJ' size='300' frame='true' align='right' caption='Bovine Trypsin' /> | ||
| + | <scene name='Sandbox_45/Bt-phillic/1'>hydrophilic residues</scene> | ||
| + | <scene name='Sandbox_45/Bt-phillic/3'>hydrophobic residues</scene> | ||
| + | |||
| + | <scene name='Sandbox_45/Bt-phillic_waters/2'>water interaction</scene> | ||
| + | |||
| + | <scene name='Sandbox_45/Bt-phillic_waters/3'>transparent</scene> | ||
| + | |||
| + | == Ligand Binding and Catalysis == | ||
<scene name='Sandbox_45/Btligand/1'>ligands</scene> | <scene name='Sandbox_45/Btligand/1'>ligands</scene> | ||
| Line 41: | Line 49: | ||
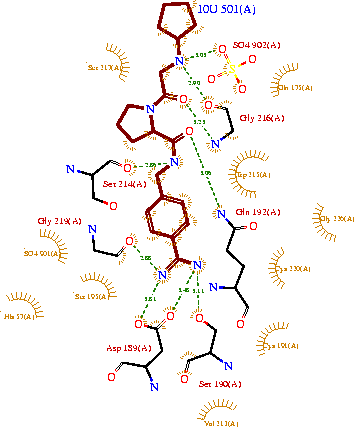
[[Image:Ligandinteractionstrypsin.gif|thumb|center|upright=2.0]] | [[Image:Ligandinteractionstrypsin.gif|thumb|center|upright=2.0]] | ||
| - | + | <scene name='Sandbox_45/Catalytic_triad/1'>catalytic triad</scene> | |
| - | + | ||
| - | <scene name='Sandbox_45/ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 01:21, 29 October 2010
Contents |
Structure of Trypsin
Primary
Bovine trypsin contains 223 amino acid residues of varied interactive tendencies and chemical characteristics, each of which contribute to the protein's structure and catalytic function.
Secondary
The spacial arrangement of hydrophobic and hydrophilic residues
sig of disulfides in overall structure, helix to beta sheet,
|
Ligand Binding and Catalysis
key amino acids