We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:David Canner/Sandbox HIV
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
===Structure of HIV-1 Protease=== | ===Structure of HIV-1 Protease=== | ||
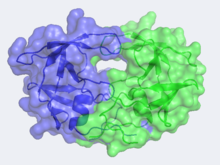
| - | Looking at the structure of HIV-1 protease, we see that the protein is composed of <scene name='User:David_Canner/Sandbox_HIV/Identical_subunits/1'>two symmetrically related subunits</scene>, each consisting of identical 99 amino acid chains. The subunits come together in such as way as to <scene name='User:David_Canner/Sandbox_HIV/Tunnel/1'>form a tunnel where they meet</scene>. This tunnel is of critical importance as it is the location where nascent proteins are bound before cleavage. In the middle of the tunnel is the active site of the protease:<scene name='User:David_Canner/Sandbox_HIV/Catalytic_triad/2'> two Asp-Thr-Gly catalytic triads</scene>. The two Asp's act as <scene name='User:David_Canner/Sandbox_HIV/Catalytic_asp/1'>the main catalytic residues</scene> in the active site and use a water molecule to help break the protein chain that binds in the tunnel. <ref>PMID:1799632</ref> You may be wondering how a protein to be cleaved makes its way into the active-site tunnel, as the<scene name='User:David_Canner/Sandbox_HIV/Hiv_tunnel_morph_length/4'> tunnel appears to be rather narrow</scene>. The key is the two flexible flaps on the top of the tunnel that can <scene name='User:David_Canner/Sandbox_HIV/Hiv_tunnel_morph/ | + | Looking at the structure of HIV-1 protease, we see that the protein is composed of <scene name='User:David_Canner/Sandbox_HIV/Identical_subunits/1'>two symmetrically related subunits</scene>, each consisting of identical 99 amino acid chains. The subunits come together in such as way as to <scene name='User:David_Canner/Sandbox_HIV/Tunnel/1'>form a tunnel where they meet</scene>. This tunnel is of critical importance as it is the location where nascent proteins are bound before cleavage. In the middle of the tunnel is the active site of the protease:<scene name='User:David_Canner/Sandbox_HIV/Catalytic_triad/2'> two Asp-Thr-Gly catalytic triads</scene>. The two Asp's act as <scene name='User:David_Canner/Sandbox_HIV/Catalytic_asp/1'>the main catalytic residues</scene> in the active site and use a water molecule to help break the protein chain that binds in the tunnel. <ref>PMID:1799632</ref> You may be wondering how a protein to be cleaved makes its way into the active-site tunnel, as the<scene name='User:David_Canner/Sandbox_HIV/Hiv_tunnel_morph_length/4'> tunnel appears to be rather narrow</scene>. The key is the two flexible flaps on the top of the tunnel that can <scene name='User:David_Canner/Sandbox_HIV/Hiv_tunnel_morph/3'>move to allow proteins </scene>to enter the tunnel. The flaps <scene name='User:David_Canner/Sandbox_HIV/Hiv_tunnel_morph_flaps/2'>undergo a dramatic movement</scene>, shifting from an open to closed conformation to securly binding its target in an appropriate conformation for cleavage. |
===Medical Implications=== | ===Medical Implications=== | ||
Revision as of 16:38, 24 November 2010
| |||||||||||
Additional Resources
For additional information, see: Human Immunodeficiency Virus
References
- ↑ Spinelli S, Liu QZ, Alzari PM, Hirel PH, Poljak RJ. The three-dimensional structure of the aspartyl protease from the HIV-1 isolate BRU. Biochimie. 1991 Nov;73(11):1391-6. PMID:1799632
- ↑ Tie Y, Kovalevsky AY, Boross P, Wang YF, Ghosh AK, Tozser J, Harrison RW, Weber IT. Atomic resolution crystal structures of HIV-1 protease and mutants V82A and I84V with saquinavir. Proteins. 2007 Apr 1;67(1):232-42. PMID:17243183 doi:10.1002/prot.21304