Poly(A) Polymerase
From Proteopedia
m (2q66 moved to Poly-A Polymerase) |
|||
| Line 1: | Line 1: | ||
| + | {{Stub}} | ||
| + | |||
[[Image:2q66.png|left|200px]] | [[Image:2q66.png|left|200px]] | ||
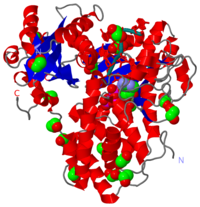
| - | + | [[Poly-A polymerase]] (polynucleotide adenylytransferase, EC 2.7.7.19) is the enzyme responsible for adding a polyadenine tail to the 3' end of a nascent pre-mRNA transcript. Its substrates are ATP and RNA. The poly(A) tail that poly-A polymerase adds to the 3' end of the pre-mRNA transcript is important for nuclear export, translation and stability of the mRNA. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Determinants of ATP Recognition== | ==Determinants of ATP Recognition== | ||
| + | <StructureSection load='2q66' scene='Proteopedia:Main_page_develop/2q66_initial/3' size='500' side='right' caption='Structure of Yeast Poly(A) Polymerase with ATP and oligo(A) (PDB entry [[2q66]]'> | ||
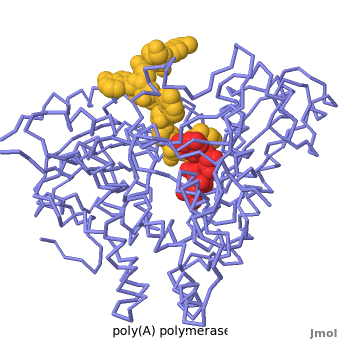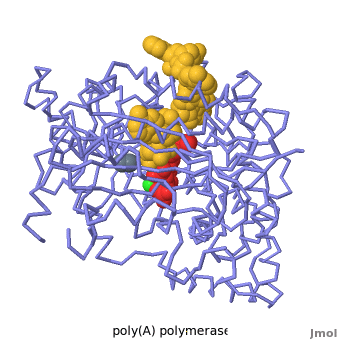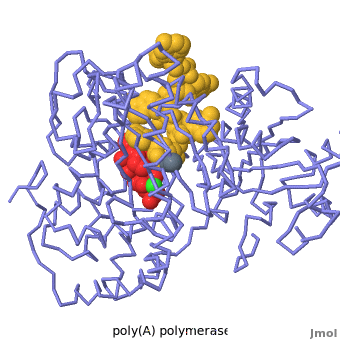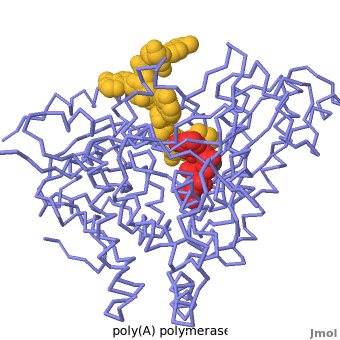
| + | [[2q66|Poly(A) polymerase]] binds specifically to ATP and adds it the end of a messenger RNA chain. This structure contains an oligo(A) polynucleotide with five nucleotides, an ATP molecule, and a magnesium ion. The enzyme is an inactive mutant with the catalytic aspartate 154 changed to alanine. Poly(A) polymerase normally has a second magnesium ion, but that second magnesium ion is absent from this structure due to the inactivating mutation D154A. In the <scene name='2q66/2q66_summary/1'>summary picture</scene>, the enzyme is in blue backbone representation, the RNA chain is in yellow, the ATP is in red, the magnesium is in green, and ALA154 is in magenta. Several mechanisms are used to achieve the specificity for ATP. The magnesium is coordinated by <scene name='2q66/2q66_asp/2'>ASP100 and ASP102</scene>, and the magnesium coordinates with the phosphates of ATP, positioning the nucleotide in the active site. The adenine base is sandwiched between the <scene name='2q66/2q66_stacking/2'>terminal base of the RNA (in yellow) and VAL234 (in cyan)</scene>. Surprisingly, there are very few contacts with the hydrogen-bonding groups in the adenine base. <scene name='2q66/2q66_asn/1'>ASN 226</scene> may form a [[hydrogen bond]] to adenine in the active enzyme, but the distance is a bit too long in this mutant structure. Instead of forming specific hydrogen bonds with the enzyme, most of the hydrogen-bonding groups in the base, sugar and phosphate interact with a shell of <scene name='Goodsell_Sandbox/2q66_water/2'>buried water molecules</scene>. Discrimination between ATP and GTP is achieved through a close steric contact between the <scene name='2q66/2q66_c2/2'>adenine C2 (in white) and the sidechains of THR 304 and MET310 (shown in cyan)</scene>. Guanine bases have an extra amino group at this position that would be too bulky to fit against these amino acids. | ||
| + | </StructureSection> | ||
| - | [[2q66|Poly(A) polymerase]] binds specifically to ATP and adds it the end of a messenger RNA chain. This structure contains an oligo(A) polynucleotide with five nucleotides, an ATP molecule, and a magnesium ion. The enzyme is an inactive mutant with the catalytic aspartate 154 changed to alanine. Poly(A) polymerase normally has a second magnesium ion, but that second magnesium ion is absent from this structure due to the inactivating mutation D154A. In the <scene name='2q66/2q66_summary/1'>summary picture</scene>, the enzyme is in blue backbone representation, the RNA chain is in yellow, the ATP is in red, the magnesium is in green, and ALA154 is in magenta. Several mechanisms are used to achieve the specificity for ATP. The magnesium is coordinated by <scene name='2q66/2q66_asp/2'>ASP100 and ASP102</scene>, and the magnesium coordinates with the phosphates of ATP, positioning the nucleotide in the active site. The adenine base is sandwiched between the <scene name='2q66/2q66_stacking/2'>terminal base of the RNA (in yellow) and VAL234 (in cyan)</scene>. Surprisingly, there are very few contacts with the hydrogen-bonding groups in the adenine base. <scene name='2q66/2q66_asn/1'>ASN 226</scene> may form a [[hydrogen bond]] to adenine in the active enzyme, but the distance is a bit too long in this mutant structure. Instead of forming specific hydrogen bonds with the enzyme, most of the hydrogen-bonding groups in the base, sugar and phosphate interact with a shell of <scene name='Goodsell_Sandbox/2q66_water/2'>buried water molecules</scene>. Discrimination between ATP and GTP is achieved through a close steric contact between the <scene name='2q66/2q66_c2/2'>adenine C2 (in white) and the sidechains of THR 304 and MET310 (shown in cyan)</scene>. Guanine bases have an extra amino group at this position that would be too bulky to fit against these amino acids. | ||
<blockquote> | <blockquote> | ||
This section complements the article on [http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb106_1.html Poly(A) Polymerase] in the | This section complements the article on [http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb106_1.html Poly(A) Polymerase] in the | ||
[http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] Series. See also [[Teaching Scenes, Tutorials, and Educators' Pages]]. | [http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] Series. See also [[Teaching Scenes, Tutorials, and Educators' Pages]]. | ||
</blockquote> | </blockquote> | ||
| - | |||
| - | <!-- | ||
| - | The line below this paragraph, {{ABSTRACT_PUBMED_17850751}}, adds the Publication Abstract to the page | ||
| - | (as it appears on PubMed at http://www.pubmed.gov), where 17850751 is the PubMed ID number. | ||
| - | --> | ||
| - | {{ABSTRACT_PUBMED_17850751}} | ||
| - | |||
| - | ==About this Structure== | ||
| - | [[2q66]] is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2Q66 OCA]. | ||
==Additional Resources== | ==Additional Resources== | ||
Revision as of 08:01, 25 November 2010
Poly-A polymerase (polynucleotide adenylytransferase, EC 2.7.7.19) is the enzyme responsible for adding a polyadenine tail to the 3' end of a nascent pre-mRNA transcript. Its substrates are ATP and RNA. The poly(A) tail that poly-A polymerase adds to the 3' end of the pre-mRNA transcript is important for nuclear export, translation and stability of the mRNA.
Determinants of ATP Recognition
| |||||||||||
This section complements the article on Poly(A) Polymerase in the Molecule of the Month Series. See also Teaching Scenes, Tutorials, and Educators' Pages.
Additional Resources
For additional information, see: Translation
Reference
Mechanism of poly(A) polymerase: structure of the enzyme-MgATP-RNA ternary complex and kinetic analysis., Balbo PB, Bohm A, Structure. 2007 Sep;15(9):1117-31. PMID:17850751
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Eran Hodis, David Canner, Joel L. Sussman, OCA, Alexander Berchansky, Jaime Prilusky, David S. Goodsell, Eric Martz