Beta-glucosidase
From Proteopedia
| Line 4: | Line 4: | ||
=== Origin and global structure of 2VRJ=== | === Origin and global structure of 2VRJ=== | ||
| - | 2VRJ is a [[β-glucosidase]] which EC number | + | 2VRJ is a [[β-glucosidase]], an '''enzyme''' which catalyses the hydrolysis of terminal non-reducing residues in β-glucosides (EC number : 3.2.1.21). It comes from ''Thermotoga maritima'' which is a rod-shaped bacterium belonging to the order of Thermotogates. This bacterium was originally isolated from geothermal heated marine sediments. |
2VRJ is here is in complex with an inhibitor called N-octyl-5-deoxy66-oxa-N-carbamoylcalystegine [1]. | 2VRJ is here is in complex with an inhibitor called N-octyl-5-deoxy66-oxa-N-carbamoylcalystegine [1]. | ||
In terms of structure 2VRJ is a homodimer. It means that it is composed of two chains <scene name='Sandbox_155/Chain_a/1'>A</scene> and <scene name='Sandbox_155/Chain_b/1'>B</scene> which are chiral. Each chain is composed of 438 residues and constitutes a subunit of the protein. Each subunit contains a''' catalytic site'''. | In terms of structure 2VRJ is a homodimer. It means that it is composed of two chains <scene name='Sandbox_155/Chain_a/1'>A</scene> and <scene name='Sandbox_155/Chain_b/1'>B</scene> which are chiral. Each chain is composed of 438 residues and constitutes a subunit of the protein. Each subunit contains a''' catalytic site'''. | ||
| - | === | + | ===Action of 2VRJ as biocatalyst=== |
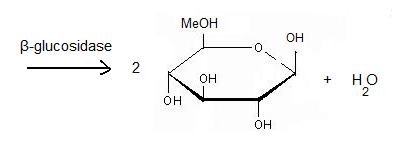
| - | + | It acts on the '''β(1-4) bond linking''' two glucose residues or glucose-substituted molecules. The action of the enzyme on such glucosides results in the release of units of glucose. For instance, hydrolysis of cellobiose catalysed by a β-glucosidase releases two glucoses [2]. | |
[[ Image:Cellobiose.jpg]][[Image:Suite.jpg]] | [[ Image:Cellobiose.jpg]][[Image:Suite.jpg]] | ||
Revision as of 08:42, 26 November 2010
Contents |
Introduction
Origin and global structure of 2VRJ
2VRJ is a β-glucosidase, an enzyme which catalyses the hydrolysis of terminal non-reducing residues in β-glucosides (EC number : 3.2.1.21). It comes from Thermotoga maritima which is a rod-shaped bacterium belonging to the order of Thermotogates. This bacterium was originally isolated from geothermal heated marine sediments. 2VRJ is here is in complex with an inhibitor called N-octyl-5-deoxy66-oxa-N-carbamoylcalystegine [1]. In terms of structure 2VRJ is a homodimer. It means that it is composed of two chains and which are chiral. Each chain is composed of 438 residues and constitutes a subunit of the protein. Each subunit contains a catalytic site.
Action of 2VRJ as biocatalyst
It acts on the β(1-4) bond linking two glucose residues or glucose-substituted molecules. The action of the enzyme on such glucosides results in the release of units of glucose. For instance, hydrolysis of cellobiose catalysed by a β-glucosidase releases two glucoses [2].
β-glucosidases can also be called β-D-glucoside glucohydrolases or cellobiases.
2VRJ
Structure and function
The enzymatic hydrolysis of a glycosidic bond requires two critical residues : a proton donor and a proton acceptor which can also be called a nucleophile/base. Aspartate and glutamate have been found to perform catalysis [3]. Accorded to this, studies showed that one of the conserved regions of β-glucosidases is centred on conserved glutamate residues [4]. As every β-glucosidase, 2VRJ presents two conserved residues of glutamate (). Moreover 2VRJ has a third important residue : [5]. The protein is presented in complex with an inhibitor called . We can see that the two glutamate residues and the asparagin are really closed to each other and to the . Such a proximity highly suggests that there are important interactions between them. So we can say that the catalytic site of 2VRJ is composed of two glutamate and one asparagin.
There are three different topologies for the active site of β-glucosidases : the pocket or crater, the cleft or groove and the tunnel [3]. The topology of 2VRJ active site is a in which the ligand can bind.
Hydrolysis of terminal non-reducing residues in β-glucosides
There are two ways to hydrolyse the terminal non-reducing residues in β-glucosides which implicate the two glutamate residues and a molecule of water [6]. Water which is an amphoter, is here used as a base for the nucleophilic attack on the positively charged anomeric carbon.
The general equation of the chemical reaction is :
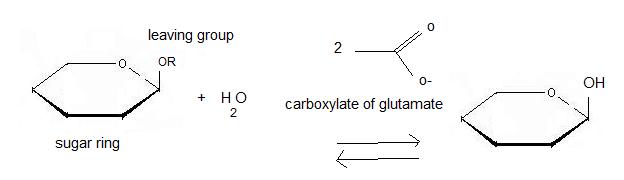
Inverting glycoside hydrolases
Inverting glycoside hydrolases lead to an inversion of the anomeric configuration to create an alpha configuration. The steps of the reaction are like the mechanism of nucleophilic substitution S2N. It is an one step process: the nucleophile (water) the anomeric carbon with simultaneous expulsion of the leaving group (OR). Bond making takes place at the same time as bond breaking. Such a mechanism is called concerted reaction. The distance between the two carboxylates is about 10.5 angströms.
Retaining glycoside hydrolases
Retaining glycoside hydrolyses occur in two steps: the first step, called glycosylation leads to the release of the leaving group and the creation of a carbocation. Subsequently, water attacks this last one. The second step, called deglycosylation consists of OR- nucleophilic attack on the intermediate and allows the deglycosylation of the enzyme. In this case, there are two transition states involved. The distance between the two carboxylates for this mechanism is about 5.5 angströms. For 2VRJ the distance between its two glutamates is about angströms. So we can say that 2VRJ seems to be a retaining enzyme.
NB: The values of the pH and the nature of the solvent play a main role in the rate of the reaction.
Glutamates are directly involved in the catalytic reaction but asparagine is used to stabilise the structure.
Other use of β-glucosidases
β-glucosidase is now used for the synthesis of biofuel. Wood is an abundant and renewable energy which can be changed into bioethanol thanks to enzymatic hydrolysis. This synthesis needs five steps. First it is pre-hydrolysis. The structure is divided into lignin and (hemi)cellulose. The cellulase can better access the structure to act on it. The second step: hydrolysis is the most important. A cellulase is a complex of 3 enzymes which act together to hydrolyse cellulose: Endoglucanase breaks the chain in the middle of the molecular structure of cellulose. Exoglucanase binds an available end of the chain and isolates it. Then units of cellobiose are cut (two units of glucose which are together). To finish, β-glucosidase divides cellobiose into two glucoses. When they ferment, they become ethanol. The final product is obtained thanks to fermentation, distillation and deshydratation.
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
[1] Aguilar M, Gloster T M, García-Moreno I M, Ortiz Mellet C, Davies G J, Llebaria A, Casas J, Egido-Gabás M, García Fernandez J M. Molecular Basis for β-Glucosidase Inhibition by Ring-Modified Calystegine Analogues. ChemBioChem 2008 ; 9 (16) : 2612-8.
[2] http://en.wikipedia.org/wiki/B-glucosidase
[3] Davies G, Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure 2004 ; 3 (9) : 853-9.
[4] http://www.ebi.ac.uk/interpro/IEntry?ac=IPR018120#PUB00002205
[5] http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/CSA/CSA_Site_Wrapper.pl?pdb=2vrj
[6] http://www.cazy.org/fam/ghf_INV_RET.html#3
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Muriel Breteau, Alexander Berchansky, Joel L. Sussman, David Canner