Beta secretase
From Proteopedia
| Line 10: | Line 10: | ||
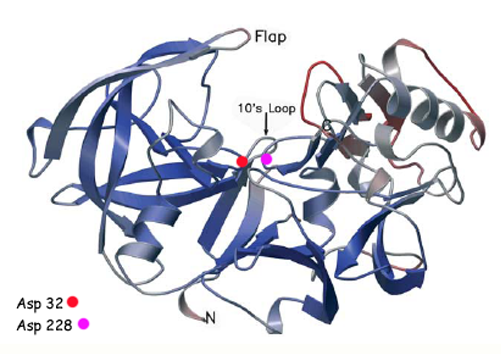
There are two other important features to β-secretase. One is the <scene name='Beta_secretase/Flap2/1'>β hairpin loop over the active site, known as the "flap"</scene>. The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor. | There are two other important features to β-secretase. One is the <scene name='Beta_secretase/Flap2/1'>β hairpin loop over the active site, known as the "flap"</scene>. The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor. | ||
| - | The other important feature is the <scene name='Beta_secretase/ | + | The other important feature is the <scene name='Beta_secretase/10sloop2/2'>10s loop made up of residues 9 through 14</scene>. The 10s loop is located in the S3 pocket of β-secretase, right between two β strands. When the 10s loop takes an open conformation, it allows for greater binding between the substrate and the S3 pocket. The 10s loop also contains within it a <scene name='Beta_secretase/10sloop2/3'>glycine residue (Gly11)</scene> that can hydrogen bond with the substrate, allowing for further stabilization of the 10s loop, as well as the overall β-secretase-substrate interaction. |
[[Image:Beta secretase2.png|center]] | [[Image:Beta secretase2.png|center]] | ||
Revision as of 03:45, 1 December 2010
|
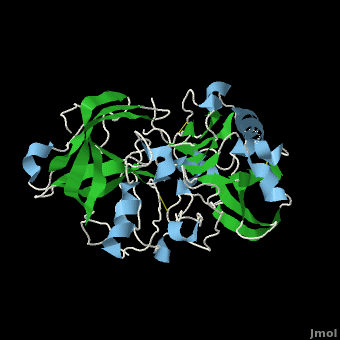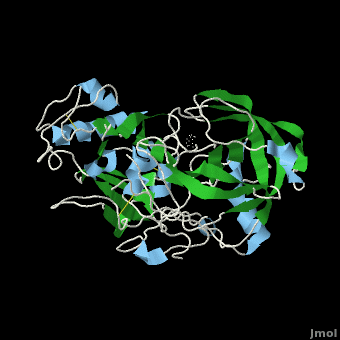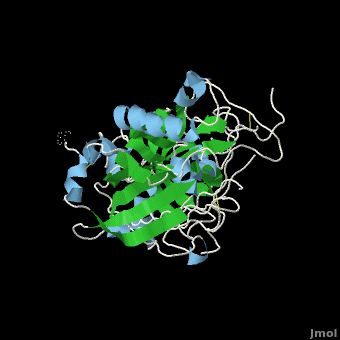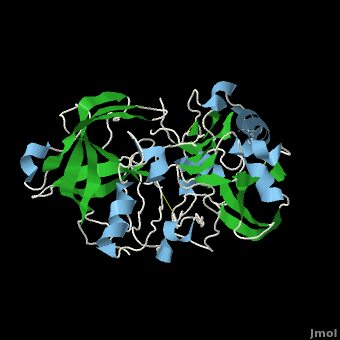
, also known as BACE (β-site of Amyloid precursor protein Cleaving Enzyme) and Memapsin 2, is an aspartyl protease found in humans. β-Secretase plays an important role in the development of Alzheimer's disease. This has made β-secretase a therapeutic target for pharmacological intervention.
Contents |
Structure of Beta Secretase
β-Secretase is a transmembrane protein, with its active site existing in the extracellular domain of the protein. Due to its nature as an aspartyl protease, β-secretase's active site is made up of . The R groups of both aspartates coordinate a single water molecule between the two of them, allowing for a nucleophilic attack to occur on the carbonyls.
There are two other important features to β-secretase. One is the . The flap is made up of residues 67 through 77. While the active site remains inactive, the flap stays in its open conformation. However, the flap is stabilized while closed over its substrate or some other inhibitor.
The other important feature is the . The 10s loop is located in the S3 pocket of β-secretase, right between two β strands. When the 10s loop takes an open conformation, it allows for greater binding between the substrate and the S3 pocket. The 10s loop also contains within it a that can hydrogen bond with the substrate, allowing for further stabilization of the 10s loop, as well as the overall β-secretase-substrate interaction.
The three parts come together to form a sort of binding pocket for β-secretase's substrates or inhibitors. Binding to the active site activates the flap to close and initiates binding by the 10s loop, all to help stabilize the structure.
Alzheimer's Disease
Alzheimer's disease is a neurodegenerative disease, and one of the most common forms of dementia. Early symptoms include things like loss of short-term memory and impairment of some physical movements. As the disease progresses, memory impairment worsens, muscle deteriorates, and the patient eventually dies. Patients of the later stages of Alzheimer's usually need to be taken care of as they are not able to take care of themselves at that point.
Alzheimer's disease occurs by the build up plaques that lead to the death of neurons. The plaques themselves are formed from the buildup of amyloid beta (Aβ). Aβ is produced by the cleavage of amyloid precursor protein (APP), an integral membrane protein found in the synapses of neurons. The cleavage of APP itself can go down one of two pathways:
A. In the first pathway, α-secretase cleaves APP somewhere within the Aβ region. This creates a fragment known as sAPPα. This fragment is beneficial to neurons as it helps to protect them.
B. In the second pathway, β-secretase cleaves APP at the N-terminus of Aβ, creating a fragment of sAPPβ. Then γ-secretase cleaves APP at the C-terminus of Aβ, which exists along the transmembrane domain of APP. At this point, Aβ is released and allowed to accumulate with other fragments, forming plaques.
β-Secretase is able to cleave Aβ at its N-terminus due to the nucleophilic attack that occurs upon the the active site of β-secretase. After the water molecule is coordinated between the carbonyls of the aspartates and the N-terminus of Aβ, the two are able to react, forcing the N-terminus to break its bond with sAPPβ.
Inhibition of Beta Secretase
|
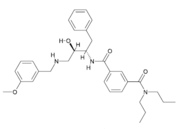
Due to β-secretase's function in the production of Aβ, it has become a very popular target for therapeutic drugs. An example of one such developed drug is OM99-2, which comes from Astex Technology.
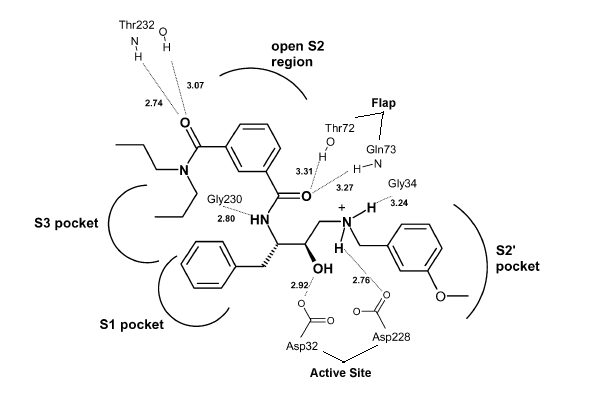
Once the inhibitor moves into place, its positively charged amine group and its hydroxyl group start to interact with β-secretase's active site. The nucelophilic attack on the aspartate's carbonyls binds OM99-2 to β-secretase. As OM99-2 becomes situated within β-secretase's binding pocket, the flap closes upon OM99-2. The flap's residues Thr72 and Gln73 bind with one of OM99-2's carbonyl groups. The 10s loop remains open to allow OM99-2 to interact with the S3 pocket. At this point .
OM99-2 remains stabilized in the pocket by binding to the other pockets of β-secretase (S1, S2, S2', and S3). Additionally, OM99-2 is able to interact with other residues in β-secretase (in this case Gly34 and Thr232), though this is not the case for all inhibitors.
References
- Alzheimer's Disease: Unraveling the Mystery. US Department of Health and Human Services, National Institute on Aging, NIH. 2008.
- CambridgeJournals. <http://journals.cambridge.org/fulltext_content/EPH/EPH87_04/S0958067001024046g003.htm>
- Patel S, Vuillard L, Cleasby A, Murray CW, Yon J (2004). "Apo and Inhibitor Complex Structures of BACE (β-secretase)". J.Mol.Biol. 343:407.
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M (1999). "β-Secretase Cleavage of Alzheimer's Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE". Science 286:735-741.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Daniel Santos, Jaime Prilusky, Joel L. Sussman, David Canner