Llama Antibody Inhibits Botulinum Neruotoxin
From Proteopedia
| Line 39: | Line 39: | ||
==Introduction== | ==Introduction== | ||
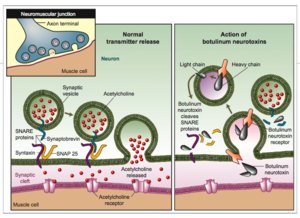
| - | Human Botulism is caused by ''Clostridium botulinum''neurotoxin(BoNT) that is characterized by flaccid muscle paralysis. Without immediate care could result in death. In addition, BoNT is also classified as one of the top six highest risk threat for bio-terrorism. BoNT has 3 domains. It is composed of a light-chain segment and two heavy-chain segments. The C-terminal portion of the heavy chain is the cell binding domain which docks the toxin to ganglioside receptors and a protein receptor on presynaptic neurons resulting in toxin endocytosis. | + | Human Botulism is caused by ''Clostridium botulinum''neurotoxin(BoNT) that is characterized by flaccid muscle paralysis. Without immediate care could result in death. In addition, BoNT is also classified as one of the top six highest risk threat for bio-terrorism. BoNT has 3 domains. It is composed of a light-chain segment and two heavy-chain segments. The C-terminal portion of the heavy chain is the cell binding domain which docks the toxin to ganglioside receptors and a protein receptor on presynaptic neurons resulting in toxin endocytosis. The translocation domain at the N-terminal portion of the heavy chain mediates escape of the toxin light chain from the endosome. The light chain is responsible for cleaving SNARE(soluble N-ethylmaleimide-sensitive factor attachment protein receptor) which is responsible for docking the synaptic vesicle, resulting in an inhibition of releasing acetylcholine. |
Revision as of 04:21, 2 December 2010
Contents |
Clostridium Botulinum
C. botulinum bacteria, which are commonly found in soil, can form spores that allow them to survive in a dormant state until they're exposed to conditions that support their growth. Infant botulism, the most common type of botulism, occurs when infants consume these spores, which then grow in their intestines and release toxin. In adults, most outbreaks are caused by contaminated home-canned foods. You can also get botulism through contaminated wounds.
If diagnosed early, botulism can be treated with an antitoxin, which blocks the action of the toxin in the blood. But recovery still takes many months. The antitoxin can also cause serious side effects, has only a very short window of application and is expensive to produce in large enough quantities to combat a bioterrorism attack. Dr. Kim Janda of The Scripps Research Institute and his colleagues, with funding from NIH's National Institute of Allergy and Infectious Diseases (NIAID) and The Skaggs Institute for Chemical Biology, set out to find other compounds that could act against botulinum toxin A.
Botulinum Toxin
Botulinum Toxin is very deadly in a sense that it prevents muscle contraction. Botulinum toxin prevents the release of Acetylcholineinto the synaptic cleft to relay the signaling to another neuron. Acetylcholine has functions both in the peripheral nervous system (PNS) and in the central nervous system (CNS) as a neuromodulator.In the peripheral nervous system, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system.In the central nervous system, acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system, which tends to cause anti-excitatory actions.
Llama Antibody Inhibits Botulinum Neruotoxin
Template:STRUCTURE 3k3q PubMed Abstract: Ingestion or inhalation of botulinum neurotoxin (BoNT) results in botulism, a severe and frequently fatal disease. Current treatments rely on antitoxins, which, while effective, cannot reverse symptoms once BoNT has entered the neuron. For treatments that can reverse intoxication, interest... [ Read More & Search PubMed Abstracts ] Ingestion or inhalation of botulinum neurotoxin (BoNT) results in botulism, a severe and frequently fatal disease. Current treatments rely on antitoxins, which, while effective, cannot reverse symptoms once BoNT has entered the neuron. For treatments that can reverse intoxication, interest has focused on developing inhibitors of the enzymatic BoNT light chain (BoNT Lc). Such inhibitors typically mimic substrate and bind in or around the substrate cleavage pocket. To explore the full range of binding sites for serotype A light chain (BoNT/A Lc) inhibitors, we created a library of non-immune llama single-domain VHH (camelid heavy-chain variable region derived from heavy-chain-only antibody) antibodies displayed on the surface of the yeast Saccharomyces cerevisiae. Library selection on BoNT/A Lc yielded 15 yeast-displayed VHH with equilibrium dissociation constants (K(d)) from 230 to 0.03 nM measured by flow cytometry. Eight of 15 VHH inhibited the cleavage of substrate SNAP25 (synaptosome-associated protein of 25,000 Da) by BoNT/A Lc. The most potent VHH (Aa1) had a solution K(d) for BoNT/A Lc of 1.47 x 10(-)(10) M and an IC(50) (50% inhibitory concentration) of 4.7 x 10(-)(10) M and was resistant to heat denaturation and reducing conditions. To understand the mechanism by which Aa1 inhibited catalysis, we solved the X-ray crystal structure of the BoNT/A Lc-Aa1 VHH complex at 2.6 A resolution. The structure reveals that the Aa1 VHH binds in the alpha-exosite of the BoNT/A Lc, far from the active site for catalysis. The study validates the utility of non-immune llama VHH libraries as a source of enzyme inhibitors and identifies the BoNT/A Lc alpha-exosite as a target for inhibitor development.
Introduction
Human Botulism is caused by Clostridium botulinumneurotoxin(BoNT) that is characterized by flaccid muscle paralysis. Without immediate care could result in death. In addition, BoNT is also classified as one of the top six highest risk threat for bio-terrorism. BoNT has 3 domains. It is composed of a light-chain segment and two heavy-chain segments. The C-terminal portion of the heavy chain is the cell binding domain which docks the toxin to ganglioside receptors and a protein receptor on presynaptic neurons resulting in toxin endocytosis. The translocation domain at the N-terminal portion of the heavy chain mediates escape of the toxin light chain from the endosome. The light chain is responsible for cleaving SNARE(soluble N-ethylmaleimide-sensitive factor attachment protein receptor) which is responsible for docking the synaptic vesicle, resulting in an inhibition of releasing acetylcholine.