Sandbox 2ju1
From Proteopedia
| Line 6: | Line 6: | ||
align='right' | align='right' | ||
caption='Acyl carrier protein DEBS'/> | caption='Acyl carrier protein DEBS'/> | ||
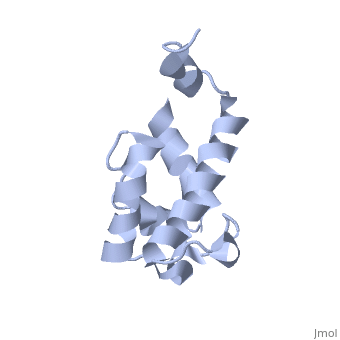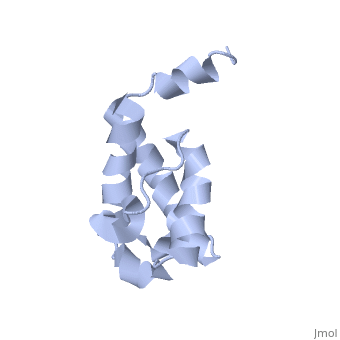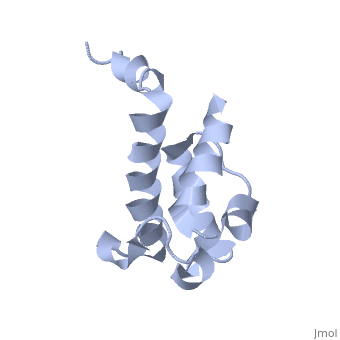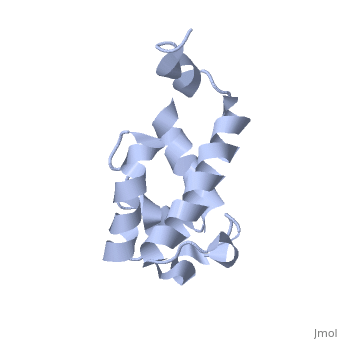
| - | Polyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. The tertiary fold of this 10-kD ACP domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. This is the ''apo''-form with the hydroxyl of serine that gets modified to ''holo''-form after post-translational modification | + | Polyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. The tertiary fold of this 10-kD ACP domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. This is the <scene name='Sandbox_2ju1/Apo-acp/1'> ''apo''- form</scene> with the hydroxyl of serine that gets modified to ''holo''-form after post-translational modification which is the active form. |
| + | <ref name="DEBS "> Cafreyy P "Identification of DEBS 1, DEBS 2 and DEBS 3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea" FEBS letters,15 June 1992, PMID:[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-44XN1WB-JW&_user=1516330&_coverDate=06/15/1992&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000053443&_version=1&_urlVersion=0&_userid=1516330&md5=31280cf1875244246efcef45bfb71fdf&searchtype=a 1618327]</ref> | ||
[http://en.wikipedia.org/wiki/Polyketide_synthase PKS] | [http://en.wikipedia.org/wiki/Polyketide_synthase PKS] | ||
<ref name="DEBS "> Cafreyy P "Identification of DEBS 1, DEBS 2 and DEBS 3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea" FEBS letters,15 June 1992, PMID:[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-44XN1WB-JW&_user=1516330&_coverDate=06/15/1992&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000053443&_version=1&_urlVersion=0&_userid=1516330&md5=31280cf1875244246efcef45bfb71fdf&searchtype=a 1618327]</ref> | <ref name="DEBS "> Cafreyy P "Identification of DEBS 1, DEBS 2 and DEBS 3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea" FEBS letters,15 June 1992, PMID:[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-44XN1WB-JW&_user=1516330&_coverDate=06/15/1992&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000053443&_version=1&_urlVersion=0&_userid=1516330&md5=31280cf1875244246efcef45bfb71fdf&searchtype=a 1618327]</ref> | ||
| - | |||
| - | <scene name='Sandbox_2ju1/Basic_residues/1'>apo form ACP</scene> | ||
Current revision
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
|
Polyketides are a medicinally important class of natural products. The architecture of modular polyketide synthases (PKSs), composed of multiple covalently linked domains grouped into modules, provides an attractive framework for engineering novel polyketide-producing assemblies. The tertiary fold of this 10-kD ACP domain is a three-helical bundle; an additional short helix in the second loop also contributes to the core helical packing. This is the with the hydroxyl of serine that gets modified to holo-form after post-translational modification which is the active form.
[1]