User:Genevieve Abbruzzese/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
| - | <scene name='User:Genevieve_Abbruzzese/ | + | <scene name='User:Genevieve_Abbruzzese/Sandbox_1/Adam13homologymodel/1'>TextToBeDisplayed</scene> |
Revision as of 04:08, 10 December 2010
- User:Genevieve Abbruzzese/Sandbox 1
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
ADAM13 is a transmembrane protein containing a metalloprotease (gray) and controls cell motility by shedding adhesion molecules from the cell surface. ADAM13 also exhibits adhesive activity through it's disintegrin (blue) and cysteine rich (purple) domains (EGF-like repeat, cyan).
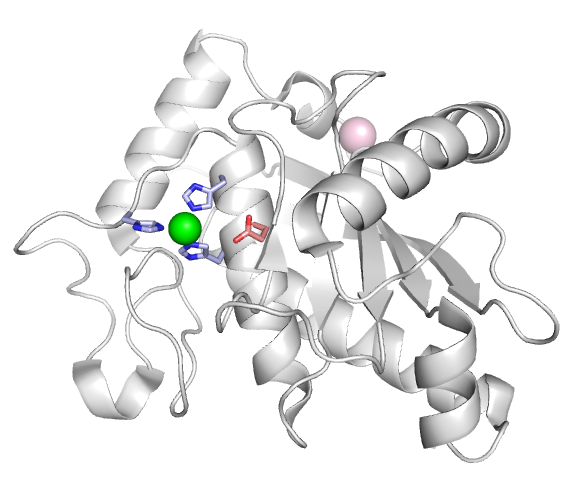
Metalloprotease domain
Most ADAMs contain a canonical metalloprotease site (HExxHxxGxxH) with a catalytic glutamate residue (shown in red) three Histidine residues (blue) coordinating a Zinc ion (green).