TolQ
From Proteopedia
| Line 1: | Line 1: | ||
| - | TolQ is a polytopic protein located in the inner (cytoplasmic) membrane. It contains approximately 230 amino acids and is important in the maintenance of the bacterial envelope integrity as well as the import of filamentous bacteriophage and group A colicins. <ref name='Vianney'> | + | TolQ is a polytopic protein located in the inner (cytoplasmic) membrane. It contains approximately 230 amino acids and is important in the maintenance of the bacterial envelope integrity as well as the import of filamentous bacteriophage and group A colicins. <ref name='Vianney'> PMID:8300535</ref> |
==Structure== | ==Structure== | ||
| Line 6: | Line 6: | ||
[[Image:TolQ.jpg|400px|right|thumb| The TolQ Membrane-spanning domains <ref>PMID: 8662905</ref>]] | [[Image:TolQ.jpg|400px|right|thumb| The TolQ Membrane-spanning domains <ref>PMID: 8662905</ref>]] | ||
| - | It has been shown that 4 to 6 TolQ molecules associate in the TolQRA complex, and that they form multimers which interact with the transmembrane helices (TMH) of TolQ, TolR and TolA <ref> PMID: 21285349</ref>. The multimers are formed by the three TMHs <ref name='Vianney'> | + | It has been shown that 4 to 6 TolQ molecules associate in the TolQRA complex, and that they form multimers which interact with the transmembrane helices (TMH) of TolQ, TolR and TolA <ref> PMID: 21285349</ref>. The multimers are formed by the three TMHs <ref name='Vianney'> PMID:8300535</ref> of TolQ, the last of which undergo a conformational change to form a hairpin, while the first TMH forms an intermolecular interaction |
==To view related Tol entries see:== | ==To view related Tol entries see:== | ||
Revision as of 15:01, 7 February 2011
TolQ is a polytopic protein located in the inner (cytoplasmic) membrane. It contains approximately 230 amino acids and is important in the maintenance of the bacterial envelope integrity as well as the import of filamentous bacteriophage and group A colicins. [1]
Structure
There are three membrane spanning segments in TolQ. TM1 is connected to TM2 via a large cytoplasmic loop, with the N-terminal ending in the periplasm.
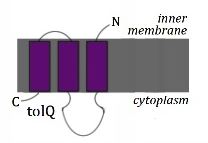
It has been shown that 4 to 6 TolQ molecules associate in the TolQRA complex, and that they form multimers which interact with the transmembrane helices (TMH) of TolQ, TolR and TolA [3]. The multimers are formed by the three TMHs [1] of TolQ, the last of which undergo a conformational change to form a hairpin, while the first TMH forms an intermolecular interaction
To view related Tol entries see:
References
- ↑ 1.0 1.1 Vianney A, Lewin TM, Beyer WF Jr, Lazzaroni JC, Portalier R, Webster RE. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J Bacteriol. 1994 Feb;176(3):822-9. PMID:8300535
- ↑ Lewin TM, Webster RE. Membrane insertion characteristics of the various transmembrane domains of the Escherichia coli TolQ protein. J Biol Chem. 1996 Jun 14;271(24):14143-9. PMID:8662905
- ↑ Zhang XY, Goemaere EL, Seddiki N, Celia H, Gavioli M, Cascales E, Lloubes R. Mapping the interaction between Escherichia coli TolQ transmembrane segments. J Biol Chem. 2011 Feb 1. PMID:21285349 doi:10.1074/jbc.M110.192773
