Citrate Synthase
From Proteopedia
m |
m (→External Resources) |
||
| Line 42: | Line 42: | ||
==External Resources== | ==External Resources== | ||
*[http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb93_1.html Citrate Synthase: September 2007 Molecule of the Month] as part of the series of tutorials that are at [http://www.pdb.org/pdb/home/home.do the RCSB Protein Data Bank] and written by [[User:David_S._Goodsell|David Goodsell]] | *[http://www.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb93_1.html Citrate Synthase: September 2007 Molecule of the Month] as part of the series of tutorials that are at [http://www.pdb.org/pdb/home/home.do the RCSB Protein Data Bank] and written by [[User:David_S._Goodsell|David Goodsell]] | ||
| - | *[http://bcs.whfreeman.com/lehninger/pages/bcs-main.asp?s=00010&n=16000&i=16010.01&v=category&o=|00510|00520|00530|00540|00550|00PRS|00010|00020|00030|00040|00050|00060|00070|00080|00090|00100|00110|00120|01000|02000|03000|04000|05000|06000|07000|08000|09000|10000|11000|12000|13000|14000|15000|16000|17000|18000|19000|20000|21000|22000|23000|24000|25000|26000|27000|28000|99000|&ns=0&t=&uid=0&rau=0 Interactive schematic animation of Citrate synthase's reaction mechanism from Lehninger's Principles of Biochemistry | + | *[http://bcs.whfreeman.com/lehninger/pages/bcs-main.asp?s=00010&n=16000&i=16010.01&v=category&o=|00510|00520|00530|00540|00550|00PRS|00010|00020|00030|00040|00050|00060|00070|00080|00090|00100|00110|00120|01000|02000|03000|04000|05000|06000|07000|08000|09000|10000|11000|12000|13000|14000|15000|16000|17000|18000|19000|20000|21000|22000|23000|24000|25000|26000|27000|28000|99000|&ns=0&t=&uid=0&rau=0 Interactive schematic animation of Citrate synthase's reaction mechanism from Lehninger's Principles of Biochemistry] |
*[http://en.wikipedia.org/wiki/Citrate_synthase Citrate synthase at wikipedia] | *[http://en.wikipedia.org/wiki/Citrate_synthase Citrate synthase at wikipedia] | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Revision as of 23:07, 20 February 2011
Contents |
The Structure and Mechanism of Citrate Synthase
|
Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme, and in fact, is often used as a enzyme marker for intact mitochondria, it is encoded by nuclear DNA, not mitochondrial [1]. The standard free energy change (ΔG°’) for the citrate synthase reaction is -31.5kJ/mol [2]. This negative free energy value means that citrate synthase is likely to function far from equilibrium under physiological conditions, and is thus a rate-determining enzyme in the citric acid cycle.
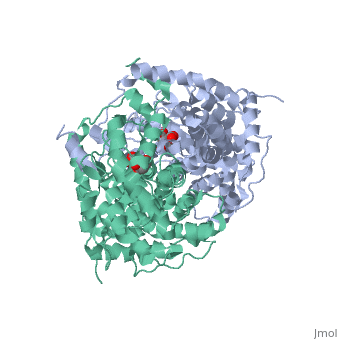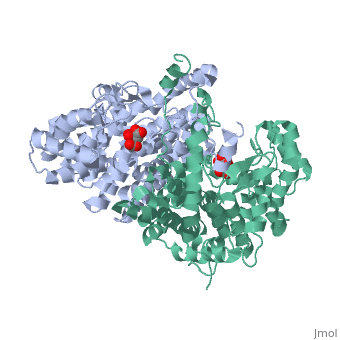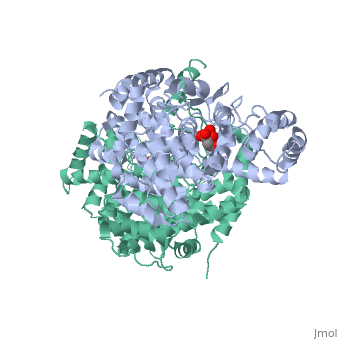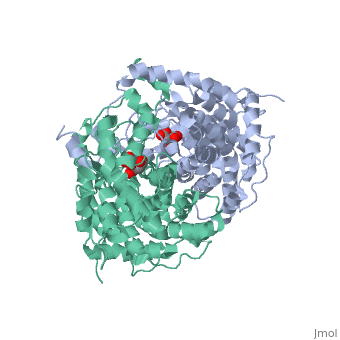
Structure: Biologically, citrate synthase exists as a
of a single amino acid chain . Each identical subunit consists of a large and a small domain, and is comprised almost entirely of α helices (making it an all α protein). In its free enzyme state, citrate synthase exists in , with its two domains forming a cleft containing the substrate (oxaloacetate) binding site (PDB: 1cts) [3][4]. When oxaloacetate binds, the smaller domain undergoes an 18° rotation, sealing the oxaloacetate binding site[5] and resulting in the (PDB: 2cts)[3]. The dramatic conformational change is best illustrated via , and be sure to view as well to get a full sense of the structural change. The conformational change not only prevents solvent from reaching the bound substrate, but also generates the acetyl-CoA binding site. This presence of “open” and “closed” forms results in citrate synthase having Ordered Sequential kinetic behavior [2].
| |||||||||||
|
|
Literature and Notes
- ↑ "Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010.
- ↑ 2.0 2.1 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.
- ↑ 3.0 3.1 3.2 Remington S, Wiegand G, Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111-52. PMID:7120407
- ↑ In this structure 1cts, citrate, the resulting product of the conversion, is actually bound where oxaloacetate binds.
- ↑ Bayer E, Bauer B, Eggerer H. Evidence from inhibitor studies for conformational changes of citrate synthase. Eur J Biochem. 1981 Nov;120(1):155-60. PMID:7308213
- ↑ Karpusas M, Branchaud B, Remington SJ. Proposed mechanism for the condensation reaction of citrate synthase: 1.9-A structure of the ternary complex with oxaloacetate and carboxymethyl coenzyme A. Biochemistry. 1990 Mar 6;29(9):2213-9. PMID:2337600
- ↑ 5cts as the state preceding condensation with oxaloacetate and a non reactive version of acetyl-CoA bound, 6cts as the state containing the bound intermediate, and [[3cts] as the complex with the products
- ↑ Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97-117. PMID:3013232 doi:http://dx.doi.org/10.1146/annurev.bb.15.060186.000525
Details of Structures Featured
1cts is a 1 chain structure with sequence from Sus scrofa. Full crystallographic information is available from OCA.
2cts is a 1 chain structure of sequence from Sus scrofa. Full crystallographic information is available from OCA.
3cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
5cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
6cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
See Also
External Resources
- Citrate Synthase: September 2007 Molecule of the Month as part of the series of tutorials that are at the RCSB Protein Data Bank and written by David Goodsell
- Interactive schematic animation of Citrate synthase's reaction mechanism from Lehninger's Principles of Biochemistry
- Citrate synthase at wikipedia
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, Michal Harel, Daniel Eddelman, Alexander Berchansky, Joel L. Sussman, Angel Herraez, David Canner, Eric Martz