Citrate Synthase
From Proteopedia
m |
m |
||
| Line 11: | Line 11: | ||
<StructureSection load='Image:5ctsBIOL.pdb.gz' size='380' side='right' scene='Citrate_Synthase/5ctsactivesite/2' caption='Citrate synthase catalysis in the closed conformation'> | <StructureSection load='Image:5ctsBIOL.pdb.gz' size='380' side='right' scene='Citrate_Synthase/5ctsactivesite/2' caption='Citrate synthase catalysis in the closed conformation'> | ||
| - | '''Mechanism:''' <scene name='Citrate_Synthase/5ctsactivesitedimer/2'>Three side chains in each of the two active sites</scene> of the dimer contribute directly to the chemistry of catalysis. Specifically in each active site when in the closed conformation, <scene name='Citrate_Synthase/5ctsactivesiteresidues/ | + | '''Mechanism:''' <scene name='Citrate_Synthase/5ctsactivesitedimer/2'>Three side chains in each of the two active sites</scene> of the dimer contribute directly to the chemistry of catalysis. Specifically in each active site when in the closed conformation, <scene name='Citrate_Synthase/5ctsactivesiteresidues/9'>these three side chains and the two substrates are together</scene> in an arrangement favorable for reaction. (By contrast, <scene name='Citrate_Synthase/Activesite1ctsto2cts/12'>the active site residues are significantly farther apart</scene> <nowiki>[</nowiki>more than ~5Å at the farthest point<nowiki>]</nowiki> in the open conformation.) {{Link Toggle AnimationOnPause}} |
| - | The reaction mechanism for citrate synthase was proposed by Remington and colleagues<ref name="1cts">PMID:7120407</ref><ref>PMID: 2337600</ref> and is illustrated here in three dimensions using structures resembling key states of the reaction<ref>[[5cts]] as the state preceding condensation with oxaloacetate and a non-reactive version of acetyl-CoA bound, [[6cts]] as the state containing the bound intermediate, and [[3cts] as the complex with the products</ref>. In this mechanism, three ionizable side chains in the active site of a monomer half of citrate synthase participate in acid-base catalysis: <scene name='Citrate_Synthase/5ctsactivesiteresidues/ | + | The reaction mechanism for citrate synthase was proposed by Remington and colleagues<ref name="1cts">PMID:7120407</ref><ref>PMID: 2337600</ref> and is illustrated here in three dimensions using structures resembling key states of the reaction<ref>[[5cts]] as the state preceding condensation with oxaloacetate and a non-reactive version of acetyl-CoA bound, [[6cts]] as the state containing the bound intermediate, and [[3cts] as the complex with the products</ref>. In this mechanism, three ionizable side chains in the active site of a monomer half of citrate synthase participate in acid-base catalysis: <scene name='Citrate_Synthase/5ctsactivesiteresidues/9'>His 274, His 320, and Asp 375</scene>. First, <scene name='Citrate_Synthase/5ctsasp375/4'>Asp 375</scene> acts as a base removing a proton from the methyl group of acetyl-CoA to form its enol. <scene name='Daniel_Eddelman_Sandbox_2/His_274/1'>His 274</scene> stabilizes the acetyl-CoA enolate by forming a hydrogen bond with the enolate oxygen. The enolate then nucleophilically attacks oxaloacetate’s carbonyl carbon, and |
<scene name='Daniel_Eddelman_Sandbox_2/His_320/1'>His 320</scene> acts as an acid donating a proton to oxaloacetate’s carbonyl group in a concerted step, forming citryl-CoA (which remains bound to the enzyme). Finally, citryl-CoA is hydrolyzed to citrate and CoA. Releasing of the CoA and the citrate involves returning to the open conformation.<br/> | <scene name='Daniel_Eddelman_Sandbox_2/His_320/1'>His 320</scene> acts as an acid donating a proton to oxaloacetate’s carbonyl group in a concerted step, forming citryl-CoA (which remains bound to the enzyme). Finally, citryl-CoA is hydrolyzed to citrate and CoA. Releasing of the CoA and the citrate involves returning to the open conformation.<br/> | ||
{{Button HeteroCPKPLUSCSColorScheme}} {{Link Toggle FancyCartoonHighQualityView}} | {{Button HeteroCPKPLUSCSColorScheme}} {{Link Toggle FancyCartoonHighQualityView}} | ||
Revision as of 03:12, 26 February 2011
Contents |
The Structure and Mechanism of Citrate Synthase
|
Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme, and in fact, is often used as a enzyme marker for intact mitochondria, it is encoded by nuclear DNA[1]. The standard free energy change (ΔG°’) for the citrate synthase reaction is -31.5kJ/mol [2]. This negative free energy value means that citrate synthase is likely to function far from equilibrium under physiological conditions, and is thus a rate-determining enzyme in the citric acid cycle.
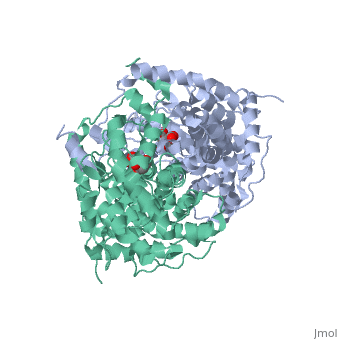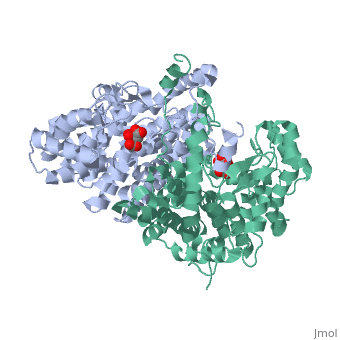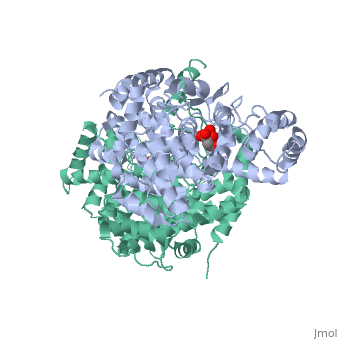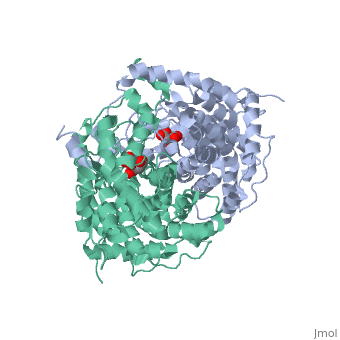
Structure: Biologically, citrate synthase exists as a
of a single amino acid chain . Each identical subunit consists of a large and a small domain, and is comprised almost entirely of α helices (making it an all α protein). In its free enzyme state, citrate synthase exists in , with its two domains forming a cleft containing the substrate (oxaloacetate) binding site (PDB: 1cts) [3][4]. When oxaloacetate binds, the smaller domain undergoes an 18° rotation, sealing the oxaloacetate binding site[5] and resulting in the (PDB: 2cts)[3]. The dramatic conformational change is best illustrated via , and be sure to view as well to get a full sense of the structural change. The conformational change not only prevents solvent from reaching the bound substrate, but also generates the acetyl-CoA binding site. This presence of “open” and “closed” forms results in citrate synthase having Ordered Sequential kinetic behavior [2].
| |||||||||||
|
|
Literature and Notes
- ↑ "Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010.
- ↑ 2.0 2.1 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.
- ↑ 3.0 3.1 3.2 Remington S, Wiegand G, Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111-52. PMID:7120407
- ↑ In this structure 1cts, citrate, the resulting product of the conversion, is actually bound where oxaloacetate binds.
- ↑ Bayer E, Bauer B, Eggerer H. Evidence from inhibitor studies for conformational changes of citrate synthase. Eur J Biochem. 1981 Nov;120(1):155-60. PMID:7308213
- ↑ Karpusas M, Branchaud B, Remington SJ. Proposed mechanism for the condensation reaction of citrate synthase: 1.9-A structure of the ternary complex with oxaloacetate and carboxymethyl coenzyme A. Biochemistry. 1990 Mar 6;29(9):2213-9. PMID:2337600
- ↑ 5cts as the state preceding condensation with oxaloacetate and a non-reactive version of acetyl-CoA bound, 6cts as the state containing the bound intermediate, and [[3cts] as the complex with the products
- ↑ Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97-117. PMID:3013232 doi:http://dx.doi.org/10.1146/annurev.bb.15.060186.000525
Details of Structures Featured
1cts is a 1 chain structure with sequence from Sus scrofa. Full crystallographic information is available from OCA.
2cts is a 1 chain structure of sequence from Sus scrofa. Full crystallographic information is available from OCA.
3cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
5cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
6cts is a 1 chain structure of sequence from Gallus gallus. Full crystallographic information is available from OCA.
See Also
External Resources
- Citrate Synthase: September 2007 Molecule of the Month as part of the series of tutorials that are at the RCSB Protein Data Bank and written by David Goodsell
- An interactive schematic animation of Citrate synthase's reaction mechanism from Lehninger's Principles of Biochemistry
- Citrate Synthase at wikipedia
Proteopedia Page Contributors and Editors (what is this?)
Wayne Decatur, Michal Harel, Daniel Eddelman, Alexander Berchansky, Joel L. Sussman, Angel Herraez, David Canner, Eric Martz