Sandbox Mati
From Proteopedia
| Line 3: | Line 3: | ||
[[ Image:1MBO_oxymyo-1.jpg|300px|left| | thumb| oxymyoglobin 1MBO]] | [[ Image:1MBO_oxymyo-1.jpg|300px|left| | thumb| oxymyoglobin 1MBO]] | ||
---- | ---- | ||
| + | ===Background=== | ||
| + | ---- | ||
| + | '''Myoglobin''' is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to [[hemoglobin]], which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood. | ||
| - | ===OVERVIEW=== | ||
| - | |||
| - | |||
| - | '''Myoglobin''' is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood. | ||
Myoglobin (abbreviated Mb) is a single-chain globular protein of 153 or 154 amino acids, containing a heme (iron-containing porphyrin) prosthetic group in the center around which the remaining apoprotein folds. It has eight alpha helices and a hydrophobic core. It has a molecular weight of 16,700 daltons, and is the primary oxygen-carrying pigment of muscle tissues. Unlike the blood-borne hemoglobin, to which it is structurally related, this protein does not exhibit cooperative binding of oxygen, since positive cooperativity is a property of multimeric/oligomeric proteins only. Instead, the binding of oxygen by myoglobin is unaffected by the oxygen pressure in the surrounding tissue. Myoglobin is often cited as having an "instant binding tenacity" to oxygen given its hyperbolic oxygen dissociation curve. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer. Diving mammals such as whales and seals have muscles with particularly high myoglobin abundance. | Myoglobin (abbreviated Mb) is a single-chain globular protein of 153 or 154 amino acids, containing a heme (iron-containing porphyrin) prosthetic group in the center around which the remaining apoprotein folds. It has eight alpha helices and a hydrophobic core. It has a molecular weight of 16,700 daltons, and is the primary oxygen-carrying pigment of muscle tissues. Unlike the blood-borne hemoglobin, to which it is structurally related, this protein does not exhibit cooperative binding of oxygen, since positive cooperativity is a property of multimeric/oligomeric proteins only. Instead, the binding of oxygen by myoglobin is unaffected by the oxygen pressure in the surrounding tissue. Myoglobin is often cited as having an "instant binding tenacity" to oxygen given its hyperbolic oxygen dissociation curve. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer. Diving mammals such as whales and seals have muscles with particularly high myoglobin abundance. | ||
| - | Myoglobin was the first protein to have its three-dimensional structure revealed. In 1958, John Kendrew and associates successfully determined the structure of myoglobin by high-resolution X-ray crystallography. For this discovery, John Kendrew shared the 1962 Nobel Prize in chemistry with Max Perutz. Despite being one of the most studied proteins in biology, its true physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin are viable, but showed a 30% reduction in cardiac systolic output. They adapted to this deficiency through hypoxic genetic mechanisms and increased vasodilation. In humans myoglobin is encoded by the MB gene. | + | Myoglobin was the first protein to have its three-dimensional structure revealed. In 1958, John Kendrew and associates successfully determined the structure of myoglobin by high-resolution[[ X-ray crystallography]]. For this discovery, John Kendrew shared the 1962 Nobel Prize in chemistry with Max Perutz. Despite being one of the most studied proteins in biology, its true physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin are viable, but showed a 30% reduction in cardiac systolic output. They adapted to this deficiency through hypoxic genetic mechanisms and increased vasodilation. In humans myoglobin is encoded by the MB gene. |
| + | ---- | ||
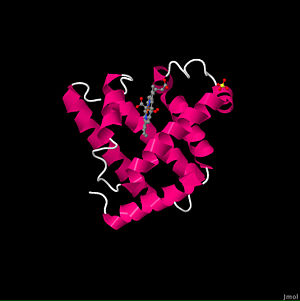
| + | ===PDB Entry=== | ||
| + | 1MBO is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Physeter_catodon Physeter catodon]. The January 2000 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Myoglobin'' by David S. Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2000_1 10.2210/rcsb_pdb/mom_2000_1]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1MBO OCA]. | ||
| + | ---- | ||
| + | ===About This Structure=== | ||
---- | ---- | ||
| - | ===STRUCTURE AND FUNCTION=== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
---- | ---- | ||
| - | === | + | ===Role in Disease=== |
| + | ---- | ||
Myoglobin is released from damaged muscle tissue (rhabdomyolysis), which has very high concentrations of myoglobin. The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may cause acute renal failure. | Myoglobin is released from damaged muscle tissue (rhabdomyolysis), which has very high concentrations of myoglobin. The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may cause acute renal failure. | ||
Myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain. However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cTnT, ECG, and clinical signs should be taken into account to make the diagnosis. | Myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain. However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cTnT, ECG, and clinical signs should be taken into account to make the diagnosis. | ||
| - | |||
---- | ---- | ||
| - | === | + | ===Reference for the Structure=== |
| - | + | <ref group="xtra">PMID:7463482</ref><references group="xtra"/> | |
| - | + | [[Category: Myoglobin]] | |
| - | + | [[Category: Physeter catodon]] | |
| - | + | [[Category: RCSB PDB Molecule of the Month]] | |
| - | + | [[Category: Phillips, S E.V.]] | |
| - | + | [[Category: Oxygen storage]] | |
| - | + | ---- | |
| - | + | ===Notes and Literature References=== | |
| - | + | ---- | |
| + | ===See Also=== | ||
| + | ---- | ||
| + | ===Additional Literature and Resources=== | ||
| + | ---- | ||
Revision as of 16:31, 3 March 2011
Contents |
MYOGLOBIN
Background
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood.
Myoglobin (abbreviated Mb) is a single-chain globular protein of 153 or 154 amino acids, containing a heme (iron-containing porphyrin) prosthetic group in the center around which the remaining apoprotein folds. It has eight alpha helices and a hydrophobic core. It has a molecular weight of 16,700 daltons, and is the primary oxygen-carrying pigment of muscle tissues. Unlike the blood-borne hemoglobin, to which it is structurally related, this protein does not exhibit cooperative binding of oxygen, since positive cooperativity is a property of multimeric/oligomeric proteins only. Instead, the binding of oxygen by myoglobin is unaffected by the oxygen pressure in the surrounding tissue. Myoglobin is often cited as having an "instant binding tenacity" to oxygen given its hyperbolic oxygen dissociation curve. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer. Diving mammals such as whales and seals have muscles with particularly high myoglobin abundance.
Myoglobin was the first protein to have its three-dimensional structure revealed. In 1958, John Kendrew and associates successfully determined the structure of myoglobin by high-resolution X-ray crystallography. For this discovery, John Kendrew shared the 1962 Nobel Prize in chemistry with Max Perutz. Despite being one of the most studied proteins in biology, its true physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin are viable, but showed a 30% reduction in cardiac systolic output. They adapted to this deficiency through hypoxic genetic mechanisms and increased vasodilation. In humans myoglobin is encoded by the MB gene.
PDB Entry
1MBO is a 1 chain structure with sequence from Physeter catodon. The January 2000 RCSB PDB Molecule of the Month feature on Myoglobin by David S. Goodsell is 10.2210/rcsb_pdb/mom_2000_1. Full crystallographic information is available from OCA.
About This Structure
Role in Disease
Myoglobin is released from damaged muscle tissue (rhabdomyolysis), which has very high concentrations of myoglobin. The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may cause acute renal failure.
Myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain. However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cTnT, ECG, and clinical signs should be taken into account to make the diagnosis.
Reference for the Structure
- Phillips SE. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531-54. PMID:7463482