This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Atropine
From Proteopedia
| Line 8: | Line 8: | ||
| - | === Introduction === | + | === Introduction and Background === |
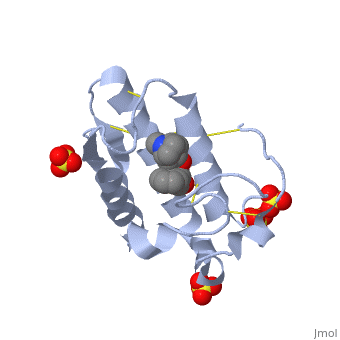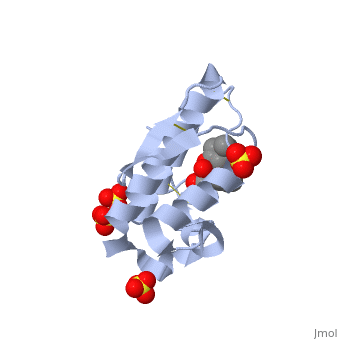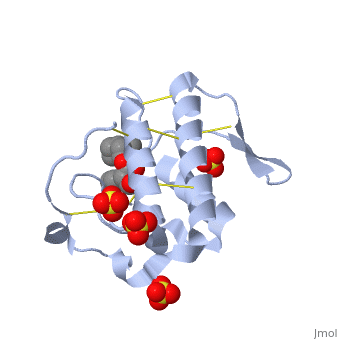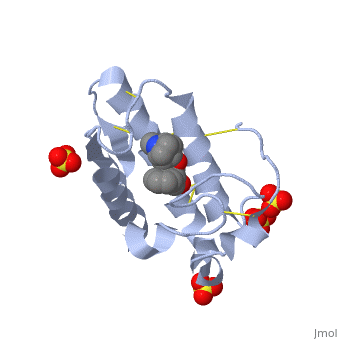
(image, atropine) | (image, atropine) | ||
| - | Atropine is an alkaloid drug derived from levohyscocyamine, a plant compound found in the family Solanaceae<ref> Atropine. Encyclopedia Brittanica. http://www.britannica.com/EBchecked/topic/42015/atropine</ref>. It is a competitive inhibitor of both acetylcholine and phospholipase 2 and has a variety of effects on both humans and animals. Although it is poisonous, in the correct dosages it has a wide variety of medical uses in both human and veterinary medicine. | + | Atropine is an alkaloid drug derived from levohyscocyamine, a plant compound found in the family Solanaceae<ref> Atropine. Encyclopedia Brittanica. http://www.britannica.com/EBchecked/topic/42015/atropine</ref>. It's chemical name is 8-methyl-8-azabicycolo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate, and the most common medicinal form of atropine is atrophine sulfate ((C17H23NO3)2·H2SO4·H2O)<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. It is a competitive inhibitor of both acetylcholine and phospholipase 2 and has a variety of effects on both humans and animals. Although it is poisonous, in the correct dosages it has a wide variety of medical uses in both human and veterinary medicine, and can be given orally, through IV fluids, or rectally. |
| - | + | ||
=== Function=== | === Function=== | ||
| Line 19: | Line 18: | ||
Atropine is part of the tropane group alkaloid family, which includes other substances such as cocaine. Atropine binds to acetylcholine receptors, blocking the action of acetylcholine and therefore suppressing the actions of the parasympathetic nervous system<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. In humans, it is metabolized approximately 50%, hydrolyzed to tropine and toropic acid, and the remaining unchanged drug is excreted in the urine<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | Atropine is part of the tropane group alkaloid family, which includes other substances such as cocaine. Atropine binds to acetylcholine receptors, blocking the action of acetylcholine and therefore suppressing the actions of the parasympathetic nervous system<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. In humans, it is metabolized approximately 50%, hydrolyzed to tropine and toropic acid, and the remaining unchanged drug is excreted in the urine<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | ||
| - | + | ==== Medical Uses ==== | |
| + | Since atropine affects the parasympathetic nervous system, it has a wide variety of effects. It is largely used as an ophthalmic drug, as it paralyzes the accommodation reflex and dilates the pupil<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. It is most commonly seen in ophthalmics, as it paralyzes the accommodation reflex in the eye and dilates the pupils. The mechanism for dilation involves blocking the contraction of the circularly pupillary sphincter muscle, which is normally stimulated by acetylcholine release<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | ||
| + | ==== Uses in Veterinary Medicine ==== | ||
| - | + | Like human medicine, atropine is widely used in veterinary medicine in a variety of different medical situations. | |
Revision as of 02:41, 8 March 2011
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
Contents |
Atropine
Introduction and Background
(image, atropine)
Atropine is an alkaloid drug derived from levohyscocyamine, a plant compound found in the family Solanaceae[1]. It's chemical name is 8-methyl-8-azabicycolo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate, and the most common medicinal form of atropine is atrophine sulfate ((C17H23NO3)2·H2SO4·H2O)[2]. It is a competitive inhibitor of both acetylcholine and phospholipase 2 and has a variety of effects on both humans and animals. Although it is poisonous, in the correct dosages it has a wide variety of medical uses in both human and veterinary medicine, and can be given orally, through IV fluids, or rectally.
Function
Atropine is part of the tropane group alkaloid family, which includes other substances such as cocaine. Atropine binds to acetylcholine receptors, blocking the action of acetylcholine and therefore suppressing the actions of the parasympathetic nervous system[3]. In humans, it is metabolized approximately 50%, hydrolyzed to tropine and toropic acid, and the remaining unchanged drug is excreted in the urine[4].
Medical Uses
Since atropine affects the parasympathetic nervous system, it has a wide variety of effects. It is largely used as an ophthalmic drug, as it paralyzes the accommodation reflex and dilates the pupil[5]. It is most commonly seen in ophthalmics, as it paralyzes the accommodation reflex in the eye and dilates the pupils. The mechanism for dilation involves blocking the contraction of the circularly pupillary sphincter muscle, which is normally stimulated by acetylcholine release[6].
Uses in Veterinary Medicine
Like human medicine, atropine is widely used in veterinary medicine in a variety of different medical situations.
|
References
- ↑ Atropine. Encyclopedia Brittanica. http://www.britannica.com/EBchecked/topic/42015/atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
Lindsey Hayes 03:53, 8 March 2011 (IST)
Proteopedia Page Contributors and Editors (what is this?)
Lindsey Hayes, David Canner, Alexander Berchansky, Michal Harel, OCA