We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Reserved Sandbox 329
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== '''Uridylyl transferases''' == | == '''Uridylyl transferases''' == | ||
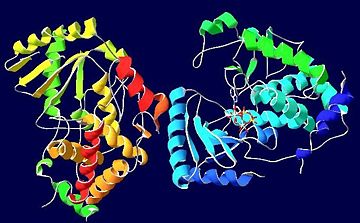
| - | + | [[Image:SECONDARY_STRUCTURE_SUCCESSION.jpg|thumb|left|upright=2.0|alt=Secondary Structure Succession of ATP-bound TUTases|Secondary structure succession of ATP-bound TUTases.]] | |
| - | [[Image:SECONDARY_STRUCTURE_SUCCESSION.jpg|thumb|left|upright=2.0|alt=Secondary Structure Succession of ATP-bound TUTases|Secondary | + | |
| - | + | ||
== INTRODUCTION == | == INTRODUCTION == | ||
| - | Terminal uridylyl transferases (TUTases) belong to a superfamily of polymerase ß nucleotidyl transferases.<ref name="primary citation">PMID:17785418</ref> TUTases have been isolated from ''Trypanosoma brucei'' and also ''Leishmania'' ssp, parasites causing diseases in humans such as African Sleeping Sickness.<ref>PMID:11893335</ref> Trypanosomal TUTases have RNA substrates that are shown to select for cognate nucleosides and provide a metal ion binding site for Mg<sup>2+</sup> ions. TUTases function in RNA editing; they add UMP to the 3' hydroxyl group.<ref name="primary citation">PMID:17785418</ref> | + | Terminal uridylyl transferases (TUTases) belong to a superfamily of polymerase ß nucleotidyl transferases.<ref name="primary citation">PMID:17785418</ref> TUTases have been isolated from ''Trypanosoma brucei'' and also ''Leishmania'' ssp, parasites causing diseases in humans such as African Sleeping Sickness.<ref>PMID:11893335</ref> TUTases function in RNA editing; more specifically they catalyze the reaction adding UMP to a RNA substrate. Trypanosomal TUTases have RNA substrates that are shown to select for cognate nucleosides and provide a metal ion binding site for Mg<sup>2+</sup> ions. TUTases function in RNA editing; they add UMP to the 3' hydroxyl group.<ref name="primary citation">PMID:17785418</ref> |
== STRUCTURE == | == STRUCTURE == | ||
| Line 15: | Line 13: | ||
The bound [[ligand]] is an <scene name='Reserved_Sandbox_329/Ligand/4'>ATP complex</scene> with two Mg<sup>2+</sup> ions. | The bound [[ligand]] is an <scene name='Reserved_Sandbox_329/Ligand/4'>ATP complex</scene> with two Mg<sup>2+</sup> ions. | ||
| - | + | <scene name='Reserved_Sandbox_329/Asp/1'>aspartate residues</scene> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== REFERENCES == | == REFERENCES == | ||
Revision as of 21:10, 16 March 2011
| |||||||||
| 2q0d, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Gene: | TUT4 (Trypanosoma brucei) | ||||||||
| Activity: | RNA uridylyltransferase, with EC number 2.7.7.52 | ||||||||
| Related: | 2ikf, 2nom | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Uridylyl transferases
INTRODUCTION
Terminal uridylyl transferases (TUTases) belong to a superfamily of polymerase ß nucleotidyl transferases.[1] TUTases have been isolated from Trypanosoma brucei and also Leishmania ssp, parasites causing diseases in humans such as African Sleeping Sickness.[2] TUTases function in RNA editing; more specifically they catalyze the reaction adding UMP to a RNA substrate. Trypanosomal TUTases have RNA substrates that are shown to select for cognate nucleosides and provide a metal ion binding site for Mg2+ ions. TUTases function in RNA editing; they add UMP to the 3' hydroxyl group.[1]
STRUCTURE
The bound ligand is an with two Mg2+ ions.
REFERENCES
- ↑ 1.0 1.1 Stagno J, Aphasizheva I, Aphasizhev R, Luecke H. Dual role of the RNA substrate in selectivity and catalysis by terminal uridylyl transferases. Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14634-9. Epub 2007 Sep 4. PMID:17785418
- ↑ Aphasizhev R, Sbicego S, Peris M, Jang SH, Aphasizheva I, Simpson AM, Rivlin A, Simpson L. Trypanosome mitochondrial 3' terminal uridylyl transferase (TUTase): the key enzyme in U-insertion/deletion RNA editing. Cell. 2002 Mar 8;108(5):637-48. PMID:11893335