This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Journal:PLoS ONE:1
From Proteopedia
(Difference between revisions)

| Line 7: | Line 7: | ||
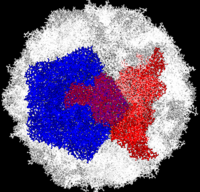
[[Image:Polio.png|left|200px|thumb|Complete Poliovirus 2 Viron based on PDB entry [[1eah]], <font color='red'><b>example of 3-fold symmetry is in red</b></font>, <font color='blue'><b>example of 5-fold symmetry is in blue</b></font> ]] | [[Image:Polio.png|left|200px|thumb|Complete Poliovirus 2 Viron based on PDB entry [[1eah]], <font color='red'><b>example of 3-fold symmetry is in red</b></font>, <font color='blue'><b>example of 5-fold symmetry is in blue</b></font> ]] | ||
| - | Poliovirus is a member of the ''Picornaviridae''. Like other members of the ''Picornaviridae'', poliovirus RNA is encapsulated in an icosahedral structure with axes of <scene name='Journal:PLoS_ONE:1/F43/1'>three-fold</scene> and <scene name='Journal:PLoS_ONE:1/F45/1'>five-fold</scene> symmetry formed from 60 capsomeres containing one copy each | + | Poliovirus is a member of the ''Picornaviridae''. Like other members of the ''Picornaviridae'', poliovirus RNA is encapsulated in an icosahedral structure with axes of <scene name='Journal:PLoS_ONE:1/F43/1'>three-fold</scene> and <scene name='Journal:PLoS_ONE:1/F45/1'>five-fold</scene> symmetry formed from 60 <scene name='Journal:PLoS_ONE:1/F1/3'>capsomeres containing one copy</scene> each of viral capsid proteins VP1 (light blue), VP2 (pale green), VP3 (light orange) and VP4(light magenta). The binding site for the human poliovirus receptor is located in a canyon at the five-fold axis of symmetry. The VP1 of picornaviruses contain a hydrophobic pocket that is accessed through this canyon. This pocket is normally occupied by pocket factors, sphingosine-like molecules including |
| - | of viral capsid proteins VP1, VP2, VP3 and VP4. The binding site for the human poliovirus receptor is located in a canyon at the five-fold axis of symmetry. The VP1 of picornaviruses contain a hydrophobic pocket that is accessed through this canyon. This pocket is normally occupied by pocket factors, sphingosine-like molecules including | + | |
palmitic and myristic acids and hydrophobic compounds, that stabilize the capsid and whose removal is a necessary prerequisite for uncoating. | palmitic and myristic acids and hydrophobic compounds, that stabilize the capsid and whose removal is a necessary prerequisite for uncoating. | ||
Revision as of 15:01, 27 March 2011
| |||||||||||
- ↑ DOI
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.