Translocation domain
From Proteopedia
| Line 3: | Line 3: | ||
[[Image: Colicin Translocation.png|500px|thumb| A diagram showing a generalised translocation path of the colicins from group A and group B into the cytoplasm, not showing all types of cytotoxic activity<ref> PMID: 21060316</ref>]] | [[Image: Colicin Translocation.png|500px|thumb| A diagram showing a generalised translocation path of the colicins from group A and group B into the cytoplasm, not showing all types of cytotoxic activity<ref> PMID: 21060316</ref>]] | ||
| + | Colicins that recruit the proteins from the Tol system are classed as being group A colicins, and those using the Ton system are group B colicins. Group A colicins also recruit a co-receptor to aid their translocation, such as [[OmpF]] or [[Tol C]]<ref> PMID: 6281233 </ref>, which is not something also seen in Group B colicins<ref> PMID: 17347522 </ref>. They may indiscriminately recruit other membrane proteins to aid translocation, that have transient interaction - this would explain why they have not yet been identified. | ||
| + | |||
| + | Once they have bound to the receptor and recruited translocation proteins, many colicins are known to unfold before moving across the membrane into the cytoplasm. However, the diameter of the unfolded peptide exceeds the diameter of the pores formed by the proteins recruited into the area<ref> PMID: 1537329 </ref><ref> PMID: 1380671 </ref>. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 17:35, 27 March 2011
The Colicin translocation domain is found at the T terminus of the colicin protein. This structure is involved in the recruitment of a group of proteins, either from the Tol system or the Ton system, after the receptor binding domain of the colicin has bound to an outer membrane receptor of the target cell, such as BtuB. The proteins recruited by the T domain are then responsible in some way for the translocation of the colicin into the cytoplasm of the target cell, through a mechanism as yet unidentified, although a few hypotheses about how this occurs do exist.
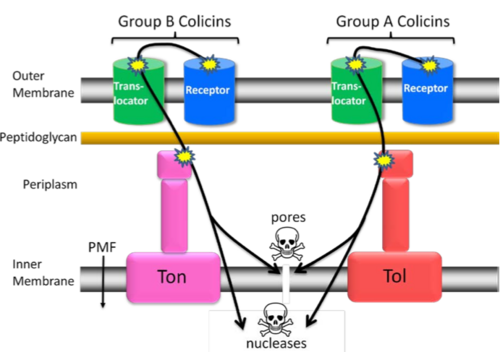
Colicins that recruit the proteins from the Tol system are classed as being group A colicins, and those using the Ton system are group B colicins. Group A colicins also recruit a co-receptor to aid their translocation, such as OmpF or Tol C[2], which is not something also seen in Group B colicins[3]. They may indiscriminately recruit other membrane proteins to aid translocation, that have transient interaction - this would explain why they have not yet been identified.
Once they have bound to the receptor and recruited translocation proteins, many colicins are known to unfold before moving across the membrane into the cytoplasm. However, the diameter of the unfolded peptide exceeds the diameter of the pores formed by the proteins recruited into the area[4][5].
References
- ↑ Kleanthous C. Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat Rev Microbiol. 2010 Dec;8(12):843-8. Epub 2010 Nov 9. PMID:21060316 doi:10.1038/nrmicro2454
- ↑ Mock M, Pugsley AP. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982 Jun;150(3):1069-76. PMID:6281233
- ↑ Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. Colicin biology. Microbiol Mol Biol Rev. 2007 Mar;71(1):158-229. PMID:17347522 doi:10.1128/MMBR.00036-06
- ↑ Benedetti H, Lloubes R, Lazdunski C, Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO J. 1992 Feb;11(2):441-7. PMID:1537329
- ↑ Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit RA, Jansonius JN, Rosenbusch JP. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727-33. PMID:1380671 doi:http://dx.doi.org/10.1038/358727a0
