Sandbox Reserved 321
From Proteopedia
| Line 18: | Line 18: | ||
The enzyme inhA is coded from the inhA gene that is simillar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]''gene which plays a role in fatty acid biosynthesis <ref name ="making drugs for inhA">PMID:5882878</ref>. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that plays a role in the sysnthesis of Mycolic Acid, and is part of a short-chain dehydrogenase/reductase family <ref name ="mech of thioamide drug action">PMID:17227913</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]''as well as other mycobateria such as ''[http://en.wikipedia.org/wiki/Mycobacterium_leprae Mycobacterium leprae]''<ref name ="TB">PMID2568869:</ref>. Inha has been propsed as the target of the thionamide drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections <ref name ="phosphorylation of inhA">PMID:21143326</ref>. | The enzyme inhA is coded from the inhA gene that is simillar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]''gene which plays a role in fatty acid biosynthesis <ref name ="making drugs for inhA">PMID:5882878</ref>. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that plays a role in the sysnthesis of Mycolic Acid, and is part of a short-chain dehydrogenase/reductase family <ref name ="mech of thioamide drug action">PMID:17227913</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]''as well as other mycobateria such as ''[http://en.wikipedia.org/wiki/Mycobacterium_leprae Mycobacterium leprae]''<ref name ="TB">PMID2568869:</ref>. Inha has been propsed as the target of the thionamide drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections <ref name ="phosphorylation of inhA">PMID:21143326</ref>. | ||
| - | ==Structure== | + | ==Structure of inhA== |
The overall strucuture of the inhA enzyme of ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]'' consists of a single domain with two substructures<ref name ="making drugs for inhA">PMID:5882878</ref>. | The overall strucuture of the inhA enzyme of ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]'' consists of a single domain with two substructures<ref name ="making drugs for inhA">PMID:5882878</ref>. | ||
| - | ===Substructure 1=== | + | ===Substructure 1 of inhA=== |
| - | Substructure one consists of 6 parallel β strands and 4 α helices interwoven together to form a core α/β structure that contains the n-terminal domain | + | Substructure one consists of 6 parallel β strands and 4 α helices interwoven together to form a core α/β structure that contains the n-terminal domain<ref name ="making drugs for inhA">PMID:5882878</ref>. |
| - | The first substructure can be further broken down into <scene name='Sandbox_Reserved_321/Substructure1section1/7'> | + | The first substructure can be further broken down into two sections, the <scene name='Sandbox_Reserved_321/Substructure1section1/7'>first section</scene> consisting of two β strands <scene name='Sandbox_Reserved_321/B-1_and_b-2/4'>(B-1 and B-2)</scene>and two short α helicies <scene name='Sandbox_Reserved_321/A-1_and_a-2/2'>(A-1 and A-2)</scene><ref name ="making drugs for inhA">PMID:5882878</ref>. |
| + | The first section is connected to the <scene name='Sandbox_Reserved_321/Section2substructure1/1'>second section</scene> by a β strand <scene name='Sandbox_Reserved_321/B-3/1'>(B-3)</scene> that crosses over the two domains, and leads into the second section initiating at the fourth α helix (A-4)</scene><ref name ="making drugs for inhA">PMID:5882878</ref>. A-4 then leads into a fifth strand β (B-5), followed by a 25 residue α helix (A-5), and finally the last strand β (B-6)<ref name ="making drugs for inhA">PMID:5882878</ref>. | ||
| + | ===Substructure 2 of inhA=== | ||
| - | + | Substructure two contains the c-terminal region of the molecule and consists of a small β strand (B-7), and two α helicies (A-6 and A-7) which are conected by a short five residue loop<ref name ="making drugs for inhA">PMID:5882878</ref>. | |
| - | + | ||
| - | + | ||
| - | + | ||
==Physiological Function== | ==Physiological Function== | ||
| + | |||
| + | ==Role in the Mycolic Acid Pathway== | ||
==Protein Superfamilly== | ==Protein Superfamilly== | ||
Revision as of 03:29, 31 March 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
InhA
by Kelly Hrywkiw
| |||||||||
| 2h9i, resolution 2.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | inhA (Mycobacterium tuberculosis) | ||||||||
| Activity: | [acyl-carrier-protein_reductase_(NADH) Enoyl-[acyl-carrier-protein] reductase (NADH)], with EC number 1.3.1.9 | ||||||||
| Related: | 1zid | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
The enzyme inhA is coded from the inhA gene that is simillar in sequence to the Salmonella typhimuriumgene which plays a role in fatty acid biosynthesis [1]. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that plays a role in the sysnthesis of Mycolic Acid, and is part of a short-chain dehydrogenase/reductase family [2][3]. Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen Mycobacterium tuberculosisas well as other mycobateria such as Mycobacterium leprae[4]. Inha has been propsed as the target of the thionamide drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections [3].
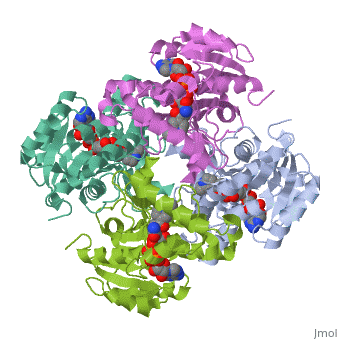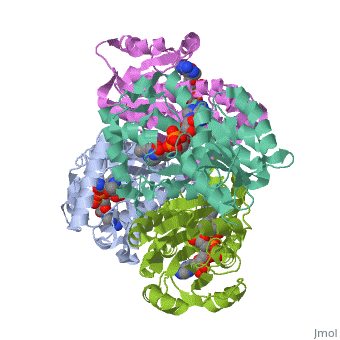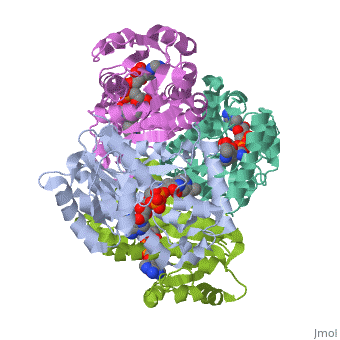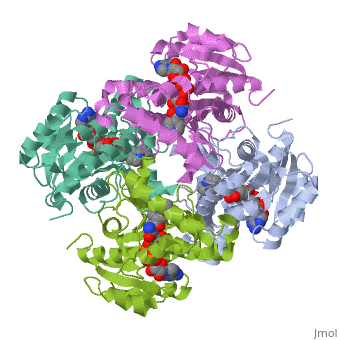
Structure of inhA
The overall strucuture of the inhA enzyme of Mycobacterium tuberculosis consists of a single domain with two substructures[1].
Substructure 1 of inhA
Substructure one consists of 6 parallel β strands and 4 α helices interwoven together to form a core α/β structure that contains the n-terminal domain[1]. The first substructure can be further broken down into two sections, the consisting of two β strands and two short α helicies [1]. The first section is connected to the by a β strand that crosses over the two domains, and leads into the second section initiating at the fourth α helix (A-4)</scene>[1]. A-4 then leads into a fifth strand β (B-5), followed by a 25 residue α helix (A-5), and finally the last strand β (B-6)[1].
Substructure 2 of inhA
Substructure two contains the c-terminal region of the molecule and consists of a small β strand (B-7), and two α helicies (A-6 and A-7) which are conected by a short five residue loop[1].
Physiological Function
Role in the Mycolic Acid Pathway
Protein Superfamilly
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Strohmaier K, Streissle G, Clemm de Noronha S. [On the determination of size of early summer meningoencephalitis]. Arch Gesamte Virusforsch. 1965;17(2):300-3. PMID:5882878
- ↑ Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR Jr, Sacchettini JC. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007 Jan 22;204(1):73-8. Epub 2007 Jan 16. PMID:17227913 doi:10.1084/jem.20062100
- ↑ 3.0 3.1 Molle V, Gulten G, Vilcheze C, Veyron-Churlet R, Zanella-Cleon I, Sacchettini JC, Jacobs WR Jr, Kremer L. Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis. Mol Microbiol. 2010 Dec;78(6):1591-605. doi:, 10.1111/j.1365-2958.2010.07446.x. Epub 2010 Nov 9. PMID:21143326 doi:10.1111/j.1365-2958.2010.07446.x
- ↑ . PMID:216315890657