Copper Amine Oxidase
From Proteopedia
| Line 15: | Line 15: | ||
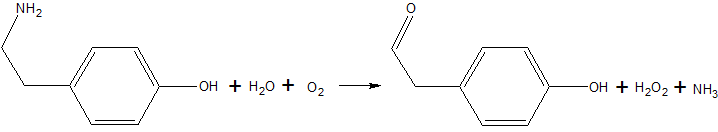
Copper amine oxidase catalyzes the oxidation of a primary amine to the corresponding aldehyde, yielding hydrogen peroxide and free ammonia. An example of this is the oxidation of [http://en.wikipedia.org/wiki/Tyramine tyramine]: | Copper amine oxidase catalyzes the oxidation of a primary amine to the corresponding aldehyde, yielding hydrogen peroxide and free ammonia. An example of this is the oxidation of [http://en.wikipedia.org/wiki/Tyramine tyramine]: | ||
| - | [[Image:Tyramine oxidation.png|The oxidation of tyramine.]] | + | [[Image:Tyramine oxidation.png|center|frame|The oxidation of tyramine, yielding the corresponding aldehyde, hydrogen peroxide, and ammonia.]] |
| + | |||
| + | |||
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 17:45, 31 March 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
Structure
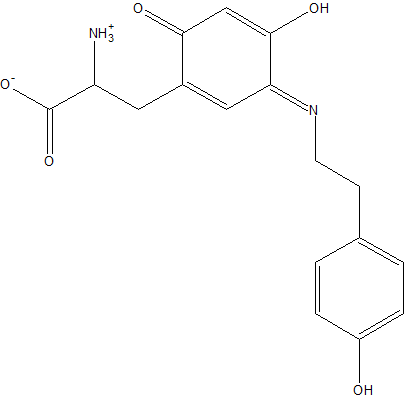
Template:STRUCTURE 2d1w 2d1w is a copper amine oxidase derived from Arthrobacter globiformis. The structure of this enzyme was determined by Murakawa et al. in 2005, by x-ray diffraction[1]. It consists of a homodimer, with each subunit containing 638 residues, one of which is a modified tyrosine residue. Each subunit also contains a copper ligand,, near the active site, which is coordinated by three histidine residues. Located near the Cu2+ ligand is a cofactor, topa quinone, both of which play a central role in the enzyme's activity[2].
Reaction
Copper amine oxidase catalyzes the oxidation of a primary amine to the corresponding aldehyde, yielding hydrogen peroxide and free ammonia. An example of this is the oxidation of tyramine:
References
- ↑ Murakawa T, Okajima T, Kuroda S, Nakamoto T, Taki M, Yamamoto Y, Hayashi H, Tanizawa K. Quantum mechanical hydrogen tunneling in bacterial copper amine oxidase reaction. Biochem Biophys Res Commun. 2006 Apr 7;342(2):414-23. Epub 2006 Feb 8. PMID:16487484 doi:10.1016/j.bbrc.2006.01.150
- ↑ Parsons MR, Convery MA, Wilmot CM, Yadav KD, Blakeley V, Corner AS, Phillips SE, McPherson MJ, Knowles PF. Crystal structure of a quinoenzyme: copper amine oxidase of Escherichia coli at 2 A resolution. Structure. 1995 Nov 15;3(11):1171-84. PMID:8591028
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Raymond Lyle, Alexander Berchansky, OCA, Jaime Prilusky