Sandbox Reserved 321
From Proteopedia
| Line 10: | Line 10: | ||
[[Image:Secondary Structure of inhA.png|thumb|left|Width200Height300|alt=Secondary Structure Succession of inhA. Secondary structure residues are ordered from blue to red.|Secondary structure succession inhA.]] | [[Image:Secondary Structure of inhA.png|thumb|left|Width200Height300|alt=Secondary Structure Succession of inhA. Secondary structure residues are ordered from blue to red.|Secondary structure succession inhA.]] | ||
__TOC__ | __TOC__ | ||
| + | |||
| + | |||
| + | |||
=Introduction= | =Introduction= | ||
| Line 40: | Line 43: | ||
| - | =Function= | + | =Function in th Mycolic Acid Pathway= |
| - | + | [[Image:Pathway.png|thumb|right|upright=2|alt=Proposed mechanism.|Formulated mechanism of Mycolic acid synthesis as proposed by Wilson et al.<ref name ="Drug Induced Alterations">PMID:10536008</ref>.]] | |
| - | = | + | InhA plays a key role in the synthesis of fatty acids, particularly in ''M. tuberculosis'' which has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together funtion in the synthesis of mycolic acids<ref name ="Function of M Tb">PMID:18552191</ref>. The final step in FASII is compleated by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation<ref name ="Function of M Tb"/><ref name ="Roles of T158">PMID:10521269</ref>. |
| - | + | Like all proteins, the specific funtion of InhA is determined by the amino acids present<ref name ="Roles of T158"/>. Specific residues that have been studied include tryrosine 158 (Y158) and lysine 165 (K165)<ref name ="Roles of T158"/>. Y158 playes an important role in alinging the carbonyl substrate, in fact; rotaion about its C -C bond by 60 brings it inot position where it can hydrogen bond to the carbonyl and provide it with electrophilic stabalization<ref name ="Roles of T158"/>. K165 is essential in the binding of the cofactor NADH, for wihout it, even at high concentrations of NADH, the reaction will not proceed<ref name ="Roles of T158"/>. | |
| Line 53: | Line 56: | ||
=References= | =References= | ||
| + | <References/> | ||
Revision as of 01:49, 1 April 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
InhA
by Kelly Hrywkiw
| |||||||||
| 2h9i, resolution 2.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | inhA (Mycobacterium tuberculosis) | ||||||||
| Activity: | [acyl-carrier-protein_reductase_(NADH) Enoyl-[acyl-carrier-protein] reductase (NADH)], with EC number 1.3.1.9 | ||||||||
| Related: | 1zid | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
The enzyme inhA is coded from the inhA gene that is simillar in sequence to the Salmonella typhimuriumgene which plays a role in fatty acid biosynthesis [1]. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that plays a role in the sysnthesis of Mycolic Acid, and is part of a short-chain dehydrogenase/reductase family [2][3]. Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen Mycobacterium tuberculosisas well as other mycobateria such as Mycobacterium leprae[4]. Inha has been propsed as the target of the thioamide drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections [3].
Structure of inhA
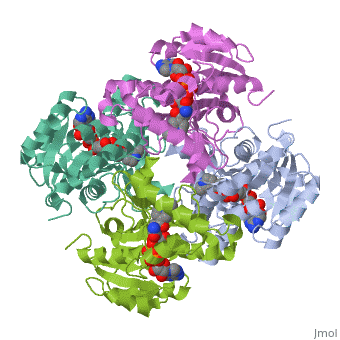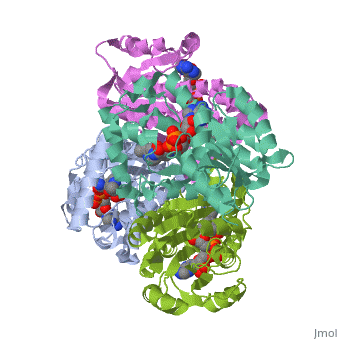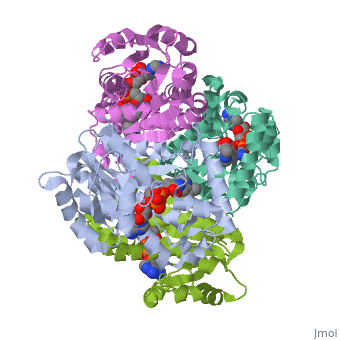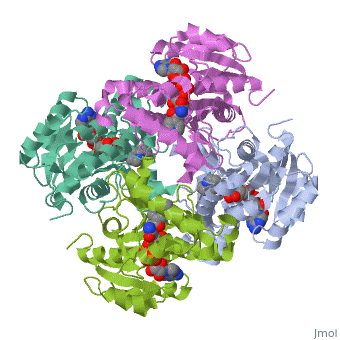
The inhA enzyme of M. tuberculosis is a homotetramer composed of a repeating subunit comprised of a single domain with a Rossmann Fold in the core that provides a NADH binding site[5]. The single domain can be broken down into two substructures that are connected by short peptide loop[1][5].
|
Substructure 1 of inhA
consists of 6 parallel β strands and 4 α helices interwoven together to form a core α/β structure that contains the n-terminal domain[1]. The first substructure can be further broken down into two sections, the consisting of two β strands and two short α helicies [1]. The first section is connected to the by a β strand that crosses over the two domains, and leads into the second section initiating at the third α helix [1](A-3) is connected by a long loop to a 14 residue β strand that then leads into the fourth α helix [1]. A-4 then leads into a fifth strand β , followed by a 25 residue α helix , and into the final strand β [1].
Substructure 2 of inhA
contains the c-terminal region of the molecule and consists of a small β strand , and two α helicies which are conected by a short five residue loop[1]. The C-terminal domain consits of two other α helicies [1].
Function in th Mycolic Acid Pathway
InhA plays a key role in the synthesis of fatty acids, particularly in M. tuberculosis which has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together funtion in the synthesis of mycolic acids[7]. The final step in FASII is compleated by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation[7][8].
Like all proteins, the specific funtion of InhA is determined by the amino acids present[8]. Specific residues that have been studied include tryrosine 158 (Y158) and lysine 165 (K165)[8]. Y158 playes an important role in alinging the carbonyl substrate, in fact; rotaion about its C -C bond by 60 brings it inot position where it can hydrogen bond to the carbonyl and provide it with electrophilic stabalization[8]. K165 is essential in the binding of the cofactor NADH, for wihout it, even at high concentrations of NADH, the reaction will not proceed[8].
Protein Superfamilly
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html
- ↑ Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR Jr, Sacchettini JC. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007 Jan 22;204(1):73-8. Epub 2007 Jan 16. PMID:17227913 doi:10.1084/jem.20062100
- ↑ 3.0 3.1 Molle V, Gulten G, Vilcheze C, Veyron-Churlet R, Zanella-Cleon I, Sacchettini JC, Jacobs WR Jr, Kremer L. Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis. Mol Microbiol. 2010 Dec;78(6):1591-605. doi:, 10.1111/j.1365-2958.2010.07446.x. Epub 2010 Nov 9. PMID:21143326 doi:10.1111/j.1365-2958.2010.07446.x
- ↑ . PMID:216315890657
- ↑ 5.0 5.1 Dias MV, Vasconcelos IB, Prado AM, Fadel V, Basso LA, de Azevedo WF Jr, Santos DS. Crystallographic studies on the binding of isonicotinyl-NAD adduct to wild-type and isoniazid resistant 2-trans-enoyl-ACP (CoA) reductase from Mycobacterium tuberculosis. J Struct Biol. 2007 Sep;159(3):369-80. Epub 2007 May 3. PMID:17588773 doi:http://dx.doi.org/10.1016/j.jsb.2007.04.009
- ↑ Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, Schoolnik GK. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12833-8. PMID:10536008
- ↑ 7.0 7.1 Gurvitz A, Hiltunen JK, Kastaniotis AJ. Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 2008 Aug;74(16):5078-85. Epub 2008 Jun 13. PMID:18552191 doi:10.1128/AEM.00655-08
- ↑ 8.0 8.1 8.2 8.3 8.4 Parikh S, Moynihan DP, Xiao G, Tonge PJ. Roles of tyrosine 158 and lysine 165 in the catalytic mechanism of InhA, the enoyl-ACP reductase from Mycobacterium tuberculosis. Biochemistry. 1999 Oct 12;38(41):13623-34. PMID:10521269