Sandbox Reserved 321
From Proteopedia
| Line 16: | Line 16: | ||
=Introduction= | =Introduction= | ||
| - | The enzyme inhA is coded from the inhA gene that is simillar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]''gene which plays a role in fatty acid biosynthesis <ref name ="making drugs for inhA">Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html</ref>. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that plays a role in the sysnthesis of [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic Acid] | + | The enzyme inhA is coded from the inhA gene that is simillar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]''gene which plays a role in fatty acid biosynthesis <ref name ="making drugs for inhA">Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html</ref>. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that is part of the fatty acid biosyntesis system, fatty acid synthase two (FASII), and plays a role in the sysnthesis of [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic Acid]<ref name ="mech of thioamide drug action">PMID:17227913</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. In addition it is part of a short-chain dehydrogenase/reductase family, and is found in the <ref name ="phosphorylation of inhA"/> Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]''as well as other mycobateria such as ''[http://en.wikipedia.org/wiki/Mycobacterium_leprae Mycobacterium leprae]''<ref name ="TB">PMID2568869:</ref>. Inha has been propsed as the target of the [http://en.wikipedia.org/wiki/Thioamidedrugs thioamide] drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections <ref name ="phosphorylation of inhA">PMID:21143326</ref>. |
| Line 47: | Line 47: | ||
[[Image:Pathway.png|thumb|right|upright=2|alt=Proposed mechanism.|Formulated mechanism of Mycolic acid synthesis as proposed by Wilson et al.<ref name ="Drug Induced Alterations">PMID:10536008</ref>.]] | [[Image:Pathway.png|thumb|right|upright=2|alt=Proposed mechanism.|Formulated mechanism of Mycolic acid synthesis as proposed by Wilson et al.<ref name ="Drug Induced Alterations">PMID:10536008</ref>.]] | ||
| - | InhA plays a key role in the synthesis of fatty acids, particularly in ''M. tuberculosis'' which has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together funtion in the synthesis of mycolic acids<ref name ="Function of M Tb">PMID:18552191</ref>. The final step in FASII is compleated by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation<ref name ="Function of M Tb"/><ref name ="Roles of T158">PMID:10521269</ref>. | + | InhA plays a key role in the synthesis of fatty acids, particularly in ''M. tuberculosis'' which has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together funtion in the synthesis of mycolic acids<ref name ="Function of M Tb">PMID:18552191</ref>. FASI synthesizes C16-18 and C24-26 fatty acids these are then sent to FASII promotes chain extention, forming long-chain meromycolic acids that are 56-64 carbons in length<ref name ="Fatty Acid Synthesis">PMID:18804030</ref>. The final step in FASII is compleated by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation<ref name ="Function of M Tb"/><ref name ="Roles of T158">PMID:10521269</ref>. The meromycolic acids undergo Claisen Condensation with a C26 fatty acid followed by reduction to a mature mycolic acid<ref name ="Fatty Acid Synthesis"/>. |
Revision as of 02:47, 1 April 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
InhA
by Kelly Hrywkiw
| |||||||||
| 2h9i, resolution 2.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| Gene: | inhA (Mycobacterium tuberculosis) | ||||||||
| Activity: | [acyl-carrier-protein_reductase_(NADH) Enoyl-[acyl-carrier-protein] reductase (NADH)], with EC number 1.3.1.9 | ||||||||
| Related: | 1zid | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Introduction
The enzyme inhA is coded from the inhA gene that is simillar in sequence to the Salmonella typhimuriumgene which plays a role in fatty acid biosynthesis [1]. Inha is an NADH dependent trans enoyl-acyl ACP carrier protein that is part of the fatty acid biosyntesis system, fatty acid synthase two (FASII), and plays a role in the sysnthesis of Mycolic Acid[2][3]. In addition it is part of a short-chain dehydrogenase/reductase family, and is found in the [3] Mycolic acids are long chain fatty acids that are essential in cell wall formation of the human pathogen Mycobacterium tuberculosisas well as other mycobateria such as Mycobacterium leprae[4]. Inha has been propsed as the target of the thioamide drugs, ethionamide (ETH) and isoniazid (INH), which have been used in treatment of mycobacterial infections [3].
Structure of inhA
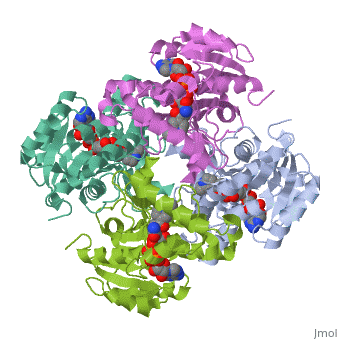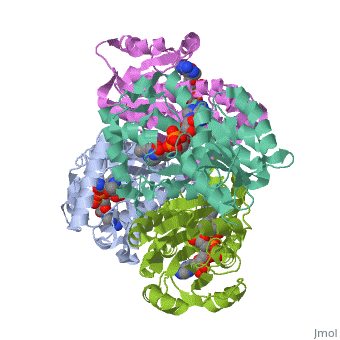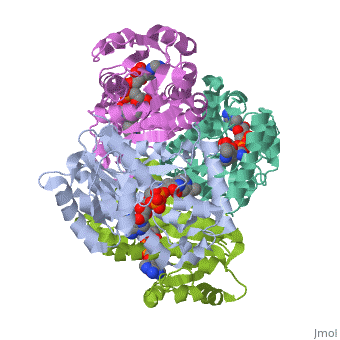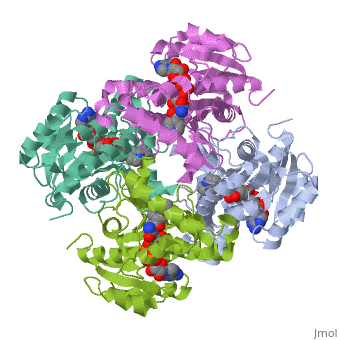
The inhA enzyme of M. tuberculosis is a homotetramer composed of a repeating subunit comprised of a single domain with a Rossmann Fold in the core that provides a NADH binding site[5]. The single domain can be broken down into two substructures that are connected by short peptide loop[1][5].
|
Substructure 1 of inhA
consists of 6 parallel β strands and 4 α helices interwoven together to form a core α/β structure that contains the n-terminal domain[1]. The first substructure can be further broken down into two sections, the consisting of two β strands and two short α helicies [1]. The first section is connected to the by a β strand that crosses over the two domains, and leads into the second section initiating at the third α helix [1](A-3) is connected by a long loop to a 14 residue β strand that then leads into the fourth α helix [1]. A-4 then leads into a fifth strand β , followed by a 25 residue α helix , and into the final strand β [1].
Substructure 2 of inhA
contains the c-terminal region of the molecule and consists of a small β strand , and two α helicies which are conected by a short five residue loop[1]. The C-terminal domain consits of two other α helicies [1].
Function in th Mycolic Acid Pathway
InhA plays a key role in the synthesis of fatty acids, particularly in M. tuberculosis which has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together funtion in the synthesis of mycolic acids[7]. FASI synthesizes C16-18 and C24-26 fatty acids these are then sent to FASII promotes chain extention, forming long-chain meromycolic acids that are 56-64 carbons in length[8]. The final step in FASII is compleated by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation[7][9]. The meromycolic acids undergo Claisen Condensation with a C26 fatty acid followed by reduction to a mature mycolic acid[8].
Like all proteins, the specific funtion of InhA is determined by the amino acids present[9]. Specific residues that have been studied include tryrosine 158 (Y158) and lysine 165 (K165)[9]. Y158 playes an important role in alinging the carbonyl substrate, in fact; rotaion about its C -C bond by 60 brings it inot position where it can hydrogen bond to the carbonyl and provide it with electrophilic stabalization[9]. K165 is essential in the binding of the cofactor NADH, for wihout it, even at high concentrations of NADH, the reaction will not proceed[9].
Protein Superfamilly
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html
- ↑ Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR Jr, Sacchettini JC. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007 Jan 22;204(1):73-8. Epub 2007 Jan 16. PMID:17227913 doi:10.1084/jem.20062100
- ↑ 3.0 3.1 3.2 Molle V, Gulten G, Vilcheze C, Veyron-Churlet R, Zanella-Cleon I, Sacchettini JC, Jacobs WR Jr, Kremer L. Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis. Mol Microbiol. 2010 Dec;78(6):1591-605. doi:, 10.1111/j.1365-2958.2010.07446.x. Epub 2010 Nov 9. PMID:21143326 doi:10.1111/j.1365-2958.2010.07446.x
- ↑ . PMID:216315890657
- ↑ 5.0 5.1 Dias MV, Vasconcelos IB, Prado AM, Fadel V, Basso LA, de Azevedo WF Jr, Santos DS. Crystallographic studies on the binding of isonicotinyl-NAD adduct to wild-type and isoniazid resistant 2-trans-enoyl-ACP (CoA) reductase from Mycobacterium tuberculosis. J Struct Biol. 2007 Sep;159(3):369-80. Epub 2007 May 3. PMID:17588773 doi:http://dx.doi.org/10.1016/j.jsb.2007.04.009
- ↑ Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, Schoolnik GK. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12833-8. PMID:10536008
- ↑ 7.0 7.1 Gurvitz A, Hiltunen JK, Kastaniotis AJ. Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 2008 Aug;74(16):5078-85. Epub 2008 Jun 13. PMID:18552191 doi:10.1128/AEM.00655-08
- ↑ 8.0 8.1 Bhatt A, Brown AK, Singh A, Minnikin DE, Besra GS. Loss of a mycobacterial gene encoding a reductase leads to an altered cell wall containing beta-oxo-mycolic acid analogs and accumulation of ketones. Chem Biol. 2008 Sep 22;15(9):930-9. PMID:18804030 doi:10.1016/j.chembiol.2008.07.007
- ↑ 9.0 9.1 9.2 9.3 9.4 Parikh S, Moynihan DP, Xiao G, Tonge PJ. Roles of tyrosine 158 and lysine 165 in the catalytic mechanism of InhA, the enoyl-ACP reductase from Mycobacterium tuberculosis. Biochemistry. 1999 Oct 12;38(41):13623-34. PMID:10521269