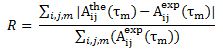Sandbox Reserved 199
From Proteopedia
| Line 53: | Line 53: | ||
Overall, the bovine Ribonuclease NMR tertiary structure matches closely the corresponding X-Ray Crystallography structure. The overall shape, main-chain fold, and side chain positions of most residues are similar between the structures of the two methods. Experimentally determined tertiary structural differences between the two methods were suggested to be due to pH differences, crystal packing, solvation, and temperature variability. | Overall, the bovine Ribonuclease NMR tertiary structure matches closely the corresponding X-Ray Crystallography structure. The overall shape, main-chain fold, and side chain positions of most residues are similar between the structures of the two methods. Experimentally determined tertiary structural differences between the two methods were suggested to be due to pH differences, crystal packing, solvation, and temperature variability. | ||
| - | Previously, researchers found the side chain position of <scene name='Sandbox_Reserved_199/2aas_-_his_119/1'>His 119</scene> in the enzyme’s active site of NMR structures to be different than that of X-Ray Crystallography studies. Crystals show a static position of this His 119 residue, yet NMR structures suggest a dynamic equilibrium between the two conformational puckers of the His 119 imidazole ring. This single residue difference between crystal and solution studies amplifies to cause a major difference in surrounding amino acid residues: 4, 106-108, and 116-118. The researchers proposed that this difference is most likely due to pH induced charge repulsion of His 119 with Asp 14 and His 48 in solution. | + | Previously, researchers found the side chain position of <scene name='Sandbox_Reserved_199/2aas_-_his_119/1'>His 119</scene> in the enzyme’s <scene name='Sandbox_Reserved_199/2aas_-_all_models/5'>active site</scene> of NMR structures to be different than that of X-Ray Crystallography studies. Crystals show a static position of this His 119 residue, yet NMR structures suggest a dynamic equilibrium between the two conformational puckers of the His 119 imidazole ring. This single residue difference between crystal and solution studies amplifies to cause a major difference in surrounding amino acid residues: 4, 106-108, and 116-118. The researchers proposed that this difference is most likely due to pH induced charge repulsion of His 119 with Asp 14 and His 48 in solution. |
More than 60 main-chain hydrogen bonds were observed, which closely corresponds to the number of hydrogen bonds determined in crystals; however, there exist a few discrepancies. In the NMR structure, there was determined to be a hydrogen bond between the amide proton on Thr 17 (NH)-Asp 14 (CO) carbonyl, as well as between Glu 49 (NH)-Val 47 (CO). The researchers suggested these differences are most likely due to the same pH phenomenon mentioned above. Other hydrogen bonds present in the NMR structure but not present in the crystal structure are: Ser 32 (NH)-Gln 28 (CO), Val 54 (NH)-Leu 51 (CO), and Cys 72 (NH)-Val 63 (CO). | More than 60 main-chain hydrogen bonds were observed, which closely corresponds to the number of hydrogen bonds determined in crystals; however, there exist a few discrepancies. In the NMR structure, there was determined to be a hydrogen bond between the amide proton on Thr 17 (NH)-Asp 14 (CO) carbonyl, as well as between Glu 49 (NH)-Val 47 (CO). The researchers suggested these differences are most likely due to the same pH phenomenon mentioned above. Other hydrogen bonds present in the NMR structure but not present in the crystal structure are: Ser 32 (NH)-Gln 28 (CO), Val 54 (NH)-Leu 51 (CO), and Cys 72 (NH)-Val 63 (CO). | ||
Revision as of 04:25, 1 April 2011
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
|
Contents |
Introduction
NMR Versus X-Ray Crystallography
The two predominant methods of protein tertiary structure determination are X-Ray Crystallographyand Nuclear Magnetic Resonance(NMR) Spectroscopy.
X-Ray Crystallography entails protein purification, crystallization of the protein, collection of X-Ray diffraction data, calculation of the protein’s electron density relative to the determined data, and finally fitting the protein’s determined residue sequence into the electron density. Crystallization of the protein often lends itself to being the most challenging aspect of this method. While any size protein can be studied via X-Ray Crystallography and the method is well-established, it is often difficult to perform for membrane proteins and the data received reveals no information about the protein’s hydrogen atoms. Also, an assumption made for X-Ray Crystallographic studies is that the crystallized protein is in a conformation similar to that seen in solution. For more information regarding X-Ray Crystallography please click here.
Bimolecular NMR Spectroscopy involves protein purification, dissolving the protein in a suitable solvent, collecting the NMR data, assigning NMR signals, and finally calculating the protein’s tertiary structure. With NMR, the most difficult step is often correctly assigning NMR signals. Although there is no need to crystallize the protein of interest and most hydrogen atoms are evident, NMR is difficult for proteins that don’t dissolve well in common solvents and works best for small proteins. 1-dimensional, 2-dimensional, and 3-dimensional NMR spectroscopy is readily available; however, 2-dimensional and 3-dimensional NMR is most often used for protein tertiary structure determination. 2D NMR reveals chemical shift correlations between spinnable nuclei such as 1H, 13C, 15N, and 13P, as well as atomic coupling, or proximity correlations via bonding. 3D NMR utilizes this methodology in addition to detection of another nuclear spin phenomenon known as the Nuclear Overhauser Effect(NOE), in which proximity correlations are can be observed in 3D space.
Due to the complexity of assigning NMR signals to specific protons, the data gathered is usually put into a computer that uses complex computer algorithms to render a protein’s tertiary structure. Even though NMR often requires more prior knowledge of the studied protein’s structural information, the end result is often more telling than that of X-Ray Crystallography. With NMR, one can gain a better insight as to what exactly a folded protein might look like in solution including the protein’s conformational flexibility. An example is the many-conformational structure of bovine pancreatic Ribonuclease seen when the page originally loads. For more information regarding NMR Spectroscopy, click here.
History of NMR Ribonuclease Studies
In 1957, the first work was published examining the structure of bovine pancreatic Ribonuclease using 1-Dimensional 1H NMR by Martin Saunder et al.
In 1988, Udgaonkar et al. used 2-dimensional 1H NMR to study bovine pancreatic Ribonuclease and examined protein folding dynamics, which supported the framework model protein folding mechanism.
NMR Study of Ribonuclease Folding Dynamics
Experimental Procedure
Using 2-dimensional 1H NMR, Udgaonkar et al. studied the folding pathway of bovine pancreatic Ribonuclease using an exchange reaction between with solvent protons. 2- dimensional 1H NMR allowed for monitoring of proton exchange in the amide backbone for ten second time intervals, and this proton labeling could be terminated via a rapid drop in pH reaction conditions. This research focused on initial protein folding steps.
Starting with denatured wt Ribonuclease, it was suggested that as the peptide began to fold, the backbone amide proteins would become less energetically favorable to exchange protons with the solvent as the backbone amide protons became involved in folding-related intermolecular interactions (such as ).
Data and Results
became evident as those involved in folding-related intermolecular interactions during initial protein folding steps: Those associated with , , , , and . All five of these protons are involved in hydrogen bonding within the of Ribonuclease; therefore, it was believed that this secondary structure was the starting point for the folding mechanism of Ribonuclease. Furthermore, this suggests the formation of a stable secondary structure before the formation of the , which is consistent with the framework model of protein folding mechanisms (in comparison with the jigsaw puzzle model).
Ribonuclease NMR Structure Versus X-Ray Crystallography Ribonuclease Structure
Experimental Procedure
Using 3-dimensional 1H NMR (2D NMR with NOE enhancement), Santoro et al. studied the tertiary structure of bovine pancreatic Ribonuclease. Their experimental raw data were processed by TRITON software, and previously determined proton assignments were utilized. The NMR tertiary structure results were categorized by their quality, or by the size and number of residual distance constraint violations. Using an equation from X-Ray Crystallography studies, the reliability factor (R-Factor) of each structure can be defined by comparing experimental NOE intensities with theoretical NOE intensities. The R-Factor can be defined as:
Where A(the) is the theoretical NOE intensity and A(exp) is the experimentally determined NOE value.
Data and Results
The NMR experiment yielded an overall R-Factor of 0.44, compared to an X-Ray Crystallographic R-Factor of 0.45. This means that the NMR structure shows a higher structural reliability compared to the X-Ray Crystallographic structure. Overall, the bovine Ribonuclease NMR tertiary structure matches closely the corresponding X-Ray Crystallography structure. The overall shape, main-chain fold, and side chain positions of most residues are similar between the structures of the two methods. Experimentally determined tertiary structural differences between the two methods were suggested to be due to pH differences, crystal packing, solvation, and temperature variability.
Previously, researchers found the side chain position of in the enzyme’s of NMR structures to be different than that of X-Ray Crystallography studies. Crystals show a static position of this His 119 residue, yet NMR structures suggest a dynamic equilibrium between the two conformational puckers of the His 119 imidazole ring. This single residue difference between crystal and solution studies amplifies to cause a major difference in surrounding amino acid residues: 4, 106-108, and 116-118. The researchers proposed that this difference is most likely due to pH induced charge repulsion of His 119 with Asp 14 and His 48 in solution.
More than 60 main-chain hydrogen bonds were observed, which closely corresponds to the number of hydrogen bonds determined in crystals; however, there exist a few discrepancies. In the NMR structure, there was determined to be a hydrogen bond between the amide proton on Thr 17 (NH)-Asp 14 (CO) carbonyl, as well as between Glu 49 (NH)-Val 47 (CO). The researchers suggested these differences are most likely due to the same pH phenomenon mentioned above. Other hydrogen bonds present in the NMR structure but not present in the crystal structure are: Ser 32 (NH)-Gln 28 (CO), Val 54 (NH)-Leu 51 (CO), and Cys 72 (NH)-Val 63 (CO).
The researchers also utilized the NMRs advantage of determining flexibility of Ribonuclease. Overall, the largest conformational flexibility resulted within the side-chains. Specifically, side-chain mobility is greatest in residues 1, 7, 15, 18, 24, 37, 59, 66, 94, 123, and 124. As expected, the backbone torsion angles were seen to be more rigid (less conformational flexibility) within the active site of Ribonuclease.
References
External Resources
Daniel Kroupa 05:03, 30 March 2011 (IST)