Ribonuclease A Catalysis
From Proteopedia
| Line 5: | Line 5: | ||
=Ribonuclease A Catalysis= | =Ribonuclease A Catalysis= | ||
==Introduction== | ==Introduction== | ||
| + | [[Image:RNaseAI.png|300px|left|thumb|Figure I: Bovine Ribonuclease A. Colored residues are representative of amino acids important to both the acid base catalysis (Red: His12 and 119) and stabilization of the transition state (Blue: Lys41 and Phe120). Figure generated via ''Pymol'' ]] | ||
| + | |||
{{STRUCTURE_7rsa | PDB=7RSA | SCENE= }} | {{STRUCTURE_7rsa | PDB=7RSA | SCENE= }} | ||
===Acid Base Catalysis=== | ===Acid Base Catalysis=== | ||
| - | Acid Base Catalysis is the acceleration of a chemical reaction by the addition of an acid or a base and is mainly used in organic chemical reactions. The acid or base is not consumed in the reaction itself. An acid transfers protons to a reactant and a base accepts protons from the reactant. An acid is often thought of as a proton and the base as a hydroxyl. When the acids or bases donate or accept protons, they stabilize the developing charges in the transition state. This usually creates a better leaving group, making the reaction more energetically favorable. Additionally, this has an effect on the activity of the nucleophile and electrophile groups. Histidine is a very common residue involved in acid-base cataylsis due to the fact that is has a pKa close to neutral; therefore, it can both accept and donate protons. | ||
| - | + | Acid Base Catalysis is the acceleration of a chemical reaction by the addition of an acid or a base and is mainly used in organic chemical reactions. It is also a common mechanism by which enzyme cause chemical changes in their substrates. The acidic or basic residues are not consumed in the reaction themselves, but there is a transfer of protons to or from the reactant depending on the nature of the residue. When the acids or bases donate or accept protons, they stabilize the developing charges in the transition state. This usually creates a better leaving group, making the reaction more energetically favorable. Additionally, this has an effect on the activity of the nucleophile and electrophile groups. Histidine is a very common residue involved in acid-base cataylsis due to the fact that is has a pKa close to neutral, pKa 6; therefore, it can both accept and donate protons at physiological pH. | |
| - | + | The acid/base mechanism of enzymes are extensively dependent on the pH of the environment and the pKa's of their residues. PKa will increase for an acidic residue if the environment is hydrophobic or if the adjacent residues are of similar charges. In the same environmental conditions, a basic residue will decrease the pKa. Having the ability to alter the pKa of certain residues such as Histidine, increases the diversity of reactions that can take place '''(reword)'''. | |
| - | The | + | |
| - | Substrate specificity of residues for RNA bases. | ||
| - | + | ===Active Site Structure=== | |
| + | The catalysis of RNA strands occurs in the active site which is the location of the chemical change in bound substrates. Subsites both up and down stream of the residues found around the active site are important for the binding of single stranded RNA. Large quantities of positively charged residues, such as Lys7 and 66 and Arg10, attract the negative charge on the phosphate back bone of the RNA strand. | ||
| + | |||
| + | The active site for RNase A actually has some specificity as to where it will degrade RNA stands. Threonine 45, located next to the active site, will hydrogen bond to pyrimidine bases, but sterically hinder the binding of a purine on the 5' strand of OH. This decreases the rates of polymeric purine strands, such as poly A, by a thousand fold. | ||
| + | |||
| + | Early Studies on RNase A showed that alkylation of His12 and 119 showed marked decrease in the catalytic activity, prompting the notion that these were the active residues in catalysis. Through mutating these residues from Histidine to the chemically unreactive alanine, reaction rates of either mutation dropped by ten-thousand fold. | ||
=='''Acid Base Catalysis by RNase A'''== | =='''Acid Base Catalysis by RNase A'''== | ||
| - | + | ===Mechanism=== | |
In the acid base catalysis of RNA in mammalians, <scene name='Sandbox_Reserved_193/Rnase_a/3'>RNase A</scene> catalyzes the cleavage of the P-O 5’ bond, and is comprised of two separate processes, the formation of the pentavalent phosphate transition state and subsequent degradation 2’3’ cyclic intermediate into its individual nucleotides. An important part of the reaction is Histidine’s ability to both accept and donate electrons. This acts as a proton source, allowing Histidine to be utilized as a base or acid, making the reaction pH dependent. | In the acid base catalysis of RNA in mammalians, <scene name='Sandbox_Reserved_193/Rnase_a/3'>RNase A</scene> catalyzes the cleavage of the P-O 5’ bond, and is comprised of two separate processes, the formation of the pentavalent phosphate transition state and subsequent degradation 2’3’ cyclic intermediate into its individual nucleotides. An important part of the reaction is Histidine’s ability to both accept and donate electrons. This acts as a proton source, allowing Histidine to be utilized as a base or acid, making the reaction pH dependent. | ||
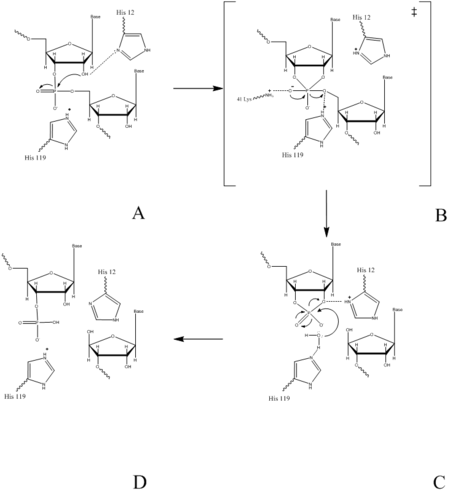
| - | [[Image:mech3.png|450px|left|thumb|Figure | + | [[Image:mech3.png|450px|left|thumb|Figure II: RNase A Catalysis. (A) Initial attack of 2'hydroxyl stabilized by His12. (B) Pentavalent phosphorous intermediate. (C) 2'3' cyclic intermediate degradation. (D) Finished products: Two distinctive nucleotide sequences. Figure generated via ''Chemdraw'']] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | The catalysis of RNase A begins when <scene name='Sandbox_Reserved_193/His12/1'>His 12</scene> undergoes basic catalysis. His12 will act as a base and abstract a proton from the RNA’s 2’ OH group; thus, assisting the attack of the 2’ oxygen on the phosphorus atom. This reaction occurs via a transition state, having a pentavalent phosphorous atom. These transition states are both stabilized by the side positive character of the side chain of <scene name='Sandbox_Reserved_193/Lys41/1'> Lys 41</scene> and the main chain of Phe120. This leads to the formation a stabilized 2’3’-cyclic intermediate. <scene name='Sandbox_Reserved_193/His119/1'>His 119</scene> will support this reaction by protonating the leaving group, the 6’ OH on the ribose of the 3’ RNA, thus acting as a general acid. | ||
| + | [[Image:Pentavalent.png|300px|right|thumb|Figure III: Pentavalent Phosphorous Transition State. Pictured borrowed from work by R. Raines]] | ||
| + | The 2’,3’- cyclic nucleotide is hydrolyzed in a separate process. His12 will donate the excess proton from the initial step to the leaving group, the 3’ oxygen of the cyclic intermediate. Simultaneously, His-119 draws the hydrogen off of a water molecule. His119 is thus reprotonated during this process, making water a better nucleophile. The water molecule attacks the phosphate causing the cleavage of the 2-3’ cyclic intermediate. The truncated nucleotide is then released with a 3’ phosphate group. | ||
| + | Upon degradation of the phosphodiester linkage between the two nucleotides the products are then released into the surrounding solvent. | ||
| + | ===Inhibitors=== | ||
| + | Due to the highly catalytic nature of RNase A for RNA strands, mammalian cells have developed a protective inhibitor to prevent pancreatic ribonucleases from degrading cystolic RNA. Ribonuclease Inhibitor (RI) tightly associates to the active site due to its non-globular nature. '''I am waiting for a paper from nature that is pertinant to RIs. If any have to due with inhibition at the active site I thought this would be a good place to add. This is what I could get from the abstract.''' | ||
Revision as of 14:46, 1 April 2011
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
Contents |
Ribonuclease A Catalysis
Introduction
| |||||||||
| 7rsa, resolution 1.26Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Pancreatic ribonuclease, with EC number 3.1.27.5 | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Acid Base Catalysis
Acid Base Catalysis is the acceleration of a chemical reaction by the addition of an acid or a base and is mainly used in organic chemical reactions. It is also a common mechanism by which enzyme cause chemical changes in their substrates. The acidic or basic residues are not consumed in the reaction themselves, but there is a transfer of protons to or from the reactant depending on the nature of the residue. When the acids or bases donate or accept protons, they stabilize the developing charges in the transition state. This usually creates a better leaving group, making the reaction more energetically favorable. Additionally, this has an effect on the activity of the nucleophile and electrophile groups. Histidine is a very common residue involved in acid-base cataylsis due to the fact that is has a pKa close to neutral, pKa 6; therefore, it can both accept and donate protons at physiological pH.
The acid/base mechanism of enzymes are extensively dependent on the pH of the environment and the pKa's of their residues. PKa will increase for an acidic residue if the environment is hydrophobic or if the adjacent residues are of similar charges. In the same environmental conditions, a basic residue will decrease the pKa. Having the ability to alter the pKa of certain residues such as Histidine, increases the diversity of reactions that can take place (reword).
Active Site Structure
The catalysis of RNA strands occurs in the active site which is the location of the chemical change in bound substrates. Subsites both up and down stream of the residues found around the active site are important for the binding of single stranded RNA. Large quantities of positively charged residues, such as Lys7 and 66 and Arg10, attract the negative charge on the phosphate back bone of the RNA strand.
The active site for RNase A actually has some specificity as to where it will degrade RNA stands. Threonine 45, located next to the active site, will hydrogen bond to pyrimidine bases, but sterically hinder the binding of a purine on the 5' strand of OH. This decreases the rates of polymeric purine strands, such as poly A, by a thousand fold.
Early Studies on RNase A showed that alkylation of His12 and 119 showed marked decrease in the catalytic activity, prompting the notion that these were the active residues in catalysis. Through mutating these residues from Histidine to the chemically unreactive alanine, reaction rates of either mutation dropped by ten-thousand fold.
Acid Base Catalysis by RNase A
Mechanism
In the acid base catalysis of RNA in mammalians, catalyzes the cleavage of the P-O 5’ bond, and is comprised of two separate processes, the formation of the pentavalent phosphate transition state and subsequent degradation 2’3’ cyclic intermediate into its individual nucleotides. An important part of the reaction is Histidine’s ability to both accept and donate electrons. This acts as a proton source, allowing Histidine to be utilized as a base or acid, making the reaction pH dependent.
The catalysis of RNase A begins when undergoes basic catalysis. His12 will act as a base and abstract a proton from the RNA’s 2’ OH group; thus, assisting the attack of the 2’ oxygen on the phosphorus atom. This reaction occurs via a transition state, having a pentavalent phosphorous atom. These transition states are both stabilized by the side positive character of the side chain of and the main chain of Phe120. This leads to the formation a stabilized 2’3’-cyclic intermediate. will support this reaction by protonating the leaving group, the 6’ OH on the ribose of the 3’ RNA, thus acting as a general acid.
The 2’,3’- cyclic nucleotide is hydrolyzed in a separate process. His12 will donate the excess proton from the initial step to the leaving group, the 3’ oxygen of the cyclic intermediate. Simultaneously, His-119 draws the hydrogen off of a water molecule. His119 is thus reprotonated during this process, making water a better nucleophile. The water molecule attacks the phosphate causing the cleavage of the 2-3’ cyclic intermediate. The truncated nucleotide is then released with a 3’ phosphate group. Upon degradation of the phosphodiester linkage between the two nucleotides the products are then released into the surrounding solvent.
Inhibitors
Due to the highly catalytic nature of RNase A for RNA strands, mammalian cells have developed a protective inhibitor to prevent pancreatic ribonucleases from degrading cystolic RNA. Ribonuclease Inhibitor (RI) tightly associates to the active site due to its non-globular nature. I am waiting for a paper from nature that is pertinant to RIs. If any have to due with inhibition at the active site I thought this would be a good place to add. This is what I could get from the abstract.
Related Sites
Works Cited
1.Raines, R. Ribonuclease A. Chemistry Review: (1998) Vol. 98 pp. 1045-1068
2.Wlodrawer, A., Svensson, L., Sjohin, L., Gilliland, G. Structure of Phosphate-Free Ribonuclease A Refined at 1.26A. Biochemistry:(1988) Vol. 27 pp. 2705-2717
Proteopedia Page Contributors and Editors (what is this?)
Nathan Clarke, David Canner, Alexander Berchansky, R. Jeremy Johnson, OCA