Sandbox Reserved 336
From Proteopedia
| Line 4: | Line 4: | ||
{{STRUCTURE_1hvy|PDB=1hvy|SCENE=}} | {{STRUCTURE_1hvy|PDB=1hvy|SCENE=}} | ||
=Thymidylate Synthase= | =Thymidylate Synthase= | ||
| - | Thymidylate Synthase is a protein found in all organisms that make DNA. Thymidylate Synthase (TS) is the essential enzyme that catalyzes the formation of dTMP from dUMP, using 5,10-methylenetetrahydrofolate (mTHF) as a cosubstrate <ref name="paper1"> | + | Thymidylate Synthase is a protein found in all organisms that make DNA. Thymidylate Synthase (TS) is the essential enzyme that catalyzes the formation of dTMP from dUMP, using 5,10-methylenetetrahydrofolate (mTHF) as a cosubstrate <ref name="paper1"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Marjorette, M., Peña, O., et al. (2011). Replacement of Val3 in Human Thymidylate Synthase Affects its Kinetic Properties and Intracellular Stability. NIH Public Access, 49(11), 2475-2482. doi: 10.1021/bi901457e.Replacement.</ref>. |
==Overview== | ==Overview== | ||
Thymidylate Synthase catalyzes the reductive methylation of deoxyuridylic acid during the de novo synthesis of thymidylic acid <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. This reaction occurs primarily during the S phase of the cell cycle. Thymidylate synthase is an essential enzyme in proliferating cells that are not supplied with an alternate source of thymidine nucleotides <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. Research has shown that thymidylate synthase enzyme levels are much higher in rapidly proliferating cells than in non dividing cells <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. | Thymidylate Synthase catalyzes the reductive methylation of deoxyuridylic acid during the de novo synthesis of thymidylic acid <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. This reaction occurs primarily during the S phase of the cell cycle. Thymidylate synthase is an essential enzyme in proliferating cells that are not supplied with an alternate source of thymidine nucleotides <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. Research has shown that thymidylate synthase enzyme levels are much higher in rapidly proliferating cells than in non dividing cells <ref name="paper2"> Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.</ref>. | ||
Revision as of 02:40, 3 April 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
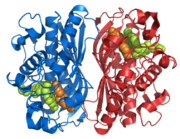
| |||||||||
| 1hvy, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Thymidylate synthase, with EC number 2.1.1.45 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Thymidylate Synthase
Thymidylate Synthase is a protein found in all organisms that make DNA. Thymidylate Synthase (TS) is the essential enzyme that catalyzes the formation of dTMP from dUMP, using 5,10-methylenetetrahydrofolate (mTHF) as a cosubstrate [1].
Overview
Thymidylate Synthase catalyzes the reductive methylation of deoxyuridylic acid during the de novo synthesis of thymidylic acid [2]. This reaction occurs primarily during the S phase of the cell cycle. Thymidylate synthase is an essential enzyme in proliferating cells that are not supplied with an alternate source of thymidine nucleotides [2]. Research has shown that thymidylate synthase enzyme levels are much higher in rapidly proliferating cells than in non dividing cells [2].
|
Mammalian Thymidylate Synthase
Human and other mammalian thymidylate synthase enzyme have a N-terminal extension of aprox 27 amino acids [3]. This extension is not present in bacterial thymidylate synthase [3]. The extension is believed to play a primary role in protein turnover however not in catalytic activity [3].
Mechanism of Thymidylate Synthase Initiation
Thymidylate synthase converts dUMP to dTMP and is labeled as the rate-limiting enzyme in the synthesis of pyrimidine nucleotides, which are required for DNA synthesis (Yamada et al., 2001). The conversion of dUMP to dTMP is done with a cosubstrate mTHF (Yamada et al., 2001). The reaction is initiated by the active site cysteine, Cys195, is attacked by C6 of dUMP and this leads to the formation of C5 of dUMP. The formation generates a second covalent bond between dUMP and mTHF and eventually a ternary catalytic complex. The role of in the conversion of dUMP to dTMP can be important to proper structure and interactions that are required to initiate the formation of C5 dUMP.
Significance
References
- ↑ Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Marjorette, M., Peña, O., et al. (2011). Replacement of Val3 in Human Thymidylate Synthase Affects its Kinetic Properties and Intracellular Stability. NIH Public Access, 49(11), 2475-2482. doi: 10.1021/bi901457e.Replacement.
- ↑ 2.0 2.1 2.2 Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.
- ↑ 3.0 3.1 3.2 Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.