Sandbox Reserved 336
From Proteopedia
| Line 12: | Line 12: | ||
Human and other mammalian thymidylate synthase enzyme have a N-terminal extension of aprox 27 amino acids <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. This extension is not present in bacterial thymidylate synthase <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. The extension is believed to play a primary role in protein turnover however not in catalytic activity <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. | Human and other mammalian thymidylate synthase enzyme have a N-terminal extension of aprox 27 amino acids <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. This extension is not present in bacterial thymidylate synthase <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. The extension is believed to play a primary role in protein turnover however not in catalytic activity <ref name="paper3"> Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.</ref>. | ||
==Mechanism of Thymidylate Synthase Initiation== | ==Mechanism of Thymidylate Synthase Initiation== | ||
| - | Thymidylate synthase converts dUMP to dTMP and is labeled as the rate-limiting enzyme in the synthesis of pyrimidine nucleotides, which are required for DNA synthesis | + | Thymidylate synthase converts dUMP to dTMP and is labeled as the rate-limiting enzyme in the synthesis of pyrimidine nucleotides, which are required for DNA synthesis <ref name="paper4"> Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.</ref>. The conversion of dUMP to dTMP is done with a cosubstrate mTHF <ref name="paper4"> Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.</ref>. The reaction is initiated by the active site cysteine, Cys195, is attacked by C6 of dUMP and this leads to the formation of C5 of dUMP <ref name="paper4"> Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.</ref>. The formation generates a second covalent bond between dUMP and mTHF and eventually a ternary catalytic complex <ref name="paper4"> Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.</ref>. The role of <scene name='Sandbox_Reserved_336/Scene_1/1'>Lipids</scene> in the conversion of dUMP to dTMP can be important to proper structure and interactions that are required to initiate the formation of C5 dUMP. |
==Significance== | ==Significance== | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 02:43, 3 April 2011
| This Sandbox is Reserved from January 10, 2010, through April 10, 2011 for use in BCMB 307-Proteins course taught by Andrea Gorrell at the University of Northern British Columbia, Prince George, BC, Canada. |
To get started:
More help: Help:Editing |
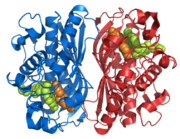
| |||||||||
| 1hvy, resolution 1.90Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Thymidylate synthase, with EC number 2.1.1.45 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Thymidylate Synthase
Thymidylate Synthase is a protein found in all organisms that make DNA. Thymidylate Synthase (TS) is the essential enzyme that catalyzes the formation of dTMP from dUMP, using 5,10-methylenetetrahydrofolate (mTHF) as a cosubstrate [1].
Overview
Thymidylate Synthase catalyzes the reductive methylation of deoxyuridylic acid during the de novo synthesis of thymidylic acid [2]. This reaction occurs primarily during the S phase of the cell cycle. Thymidylate synthase is an essential enzyme in proliferating cells that are not supplied with an alternate source of thymidine nucleotides [2]. Research has shown that thymidylate synthase enzyme levels are much higher in rapidly proliferating cells than in non dividing cells [2].
|
Mammalian Thymidylate Synthase
Human and other mammalian thymidylate synthase enzyme have a N-terminal extension of aprox 27 amino acids [3]. This extension is not present in bacterial thymidylate synthase [3]. The extension is believed to play a primary role in protein turnover however not in catalytic activity [3].
Mechanism of Thymidylate Synthase Initiation
Thymidylate synthase converts dUMP to dTMP and is labeled as the rate-limiting enzyme in the synthesis of pyrimidine nucleotides, which are required for DNA synthesis [4]. The conversion of dUMP to dTMP is done with a cosubstrate mTHF [4]. The reaction is initiated by the active site cysteine, Cys195, is attacked by C6 of dUMP and this leads to the formation of C5 of dUMP [4]. The formation generates a second covalent bond between dUMP and mTHF and eventually a ternary catalytic complex [4]. The role of in the conversion of dUMP to dTMP can be important to proper structure and interactions that are required to initiate the formation of C5 dUMP.
Significance
References
- ↑ Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Marjorette, M., Peña, O., et al. (2011). Replacement of Val3 in Human Thymidylate Synthase Affects its Kinetic Properties and Intracellular Stability. NIH Public Access, 49(11), 2475-2482. doi: 10.1021/bi901457e.Replacement.
- ↑ 2.0 2.1 2.2 Jenh, C. H., Rao, L. G., & Johnson, L. F. (1985). Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. Journal of cellular physiology, 122(1), 149-54. doi: 10.1002/jcp.1041220122.
- ↑ 3.0 3.1 3.2 Huang, X., Gibson, L. M., Bell, B. J., Lovelace, L. L., Peña, M. M. O., Berger, F. G., et al. (2010). Replacement of Val3 in human thymidylate synthase affects its kinetic properties and intracellular stability . Biochemistry, 49(11), 2475-82. doi: 10.1021/bi901457e.
- ↑ 4.0 4.1 4.2 4.3 Yamada, H., Ichikawa, W., Uetake, H., Shirota, Y., Nihei, Z., Sugihara, K., et al. (2001). Thymidylate synthase gene expression in primary colorectal cancer and metastatic sites. Clinical colorectal cancer, 1(3), 169-73; discussion 174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450430.