Cassady sandbox1
From Proteopedia
(→Regulation) |
(→Regulation) |
||
| Line 19: | Line 19: | ||
Glycolysis is an essential metabolic process for survival. Therefore, in its activation and suppression it must be highly regulated. Three points in the process of glycolysis occur with a large negative free energy and are therefore, irreversible. These three points are hexokinase, phosphofructokinase, and pyruvate kinase. These three reactions are candidates to be the major points of regulation, because they are they committed steps. Of the three PFK is considered the major regulatory point for glycolysis in muscle with a ΔG= -25.9 kJ/mol. <ref>Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.</ref>. This makes sense seeing as pyruvate kinase catalyzes the final reaction and hexokinase is an enzyme involved in more processes than glycolysis. | Glycolysis is an essential metabolic process for survival. Therefore, in its activation and suppression it must be highly regulated. Three points in the process of glycolysis occur with a large negative free energy and are therefore, irreversible. These three points are hexokinase, phosphofructokinase, and pyruvate kinase. These three reactions are candidates to be the major points of regulation, because they are they committed steps. Of the three PFK is considered the major regulatory point for glycolysis in muscle with a ΔG= -25.9 kJ/mol. <ref>Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.</ref>. This makes sense seeing as pyruvate kinase catalyzes the final reaction and hexokinase is an enzyme involved in more processes than glycolysis. | ||
| - | PFK is regulated by ATP, AMP, ADP. While ATP binds at the active site | + | PFK is regulated by ATP, AMP, ADP. While ATP binds at the active site equally well in both R and T states, it preferentially binds the allosteric site of the T state <ref>Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.</ref> This preferential binding causes a shift from equilibrium of the two states, to a greater amount of T state <ref>PubMed:2136935</ref>, which decreases the affinity for F6P. Allosteric activator <scene name='Zach_Westrick_Sandbox/Allosteric_activator/2'>ADP</scene> also binds to allosteric site to increase the ratio of R state phosphofructokinase. |
The system of regulation matches perfectly with the function of PFK. When PFK is active, ATP is being produced down stream from it as glucose is eventually broken down completely. Thus, when ATP levels are low and more needs to be made, the activity of PFK will be increased, because ADP will be in high concentration. The opposite holds true as well, when ATP is in high concentration and inhibits protein activity. | The system of regulation matches perfectly with the function of PFK. When PFK is active, ATP is being produced down stream from it as glucose is eventually broken down completely. Thus, when ATP levels are low and more needs to be made, the activity of PFK will be increased, because ADP will be in high concentration. The opposite holds true as well, when ATP is in high concentration and inhibits protein activity. | ||
Revision as of 18:56, 12 April 2011
| |||||||||
| 1pfk, resolution 2.40Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | 6-phosphofructokinase, with EC number 2.7.1.11 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
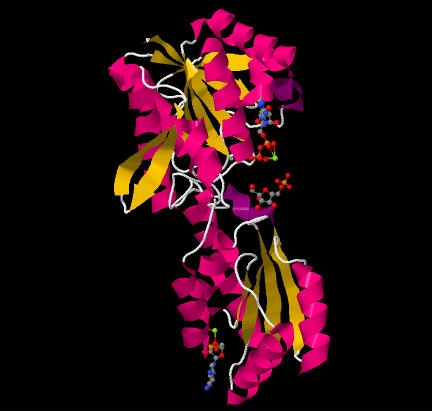
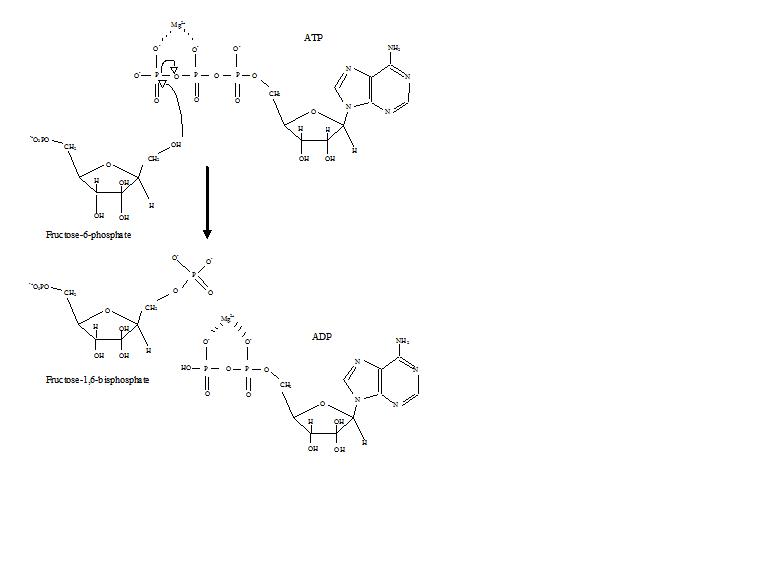
Phosphofructokinase (PFK) (PDB id 4pfk) is an approximately 300 residue enzyme that catalyzes the phosphorylation of Fructose-6-phosphate (F6P) to Fructose-1,6-bisphosphate (F1,6P) in the third reaction of glycolysis. This enzyme, with four subunits, catalyzes the most highly regulated reaction of glycolysis. Phosphofructokinase is not only the enzymes name, but also, the fold, superfamily, and family classification name.
Contents |
Role in Glycolysis
Glycolysis is the process of breaking down glucose to make pyruvic acid, which is used in anaerobic respiration or as one of the starting reactants in the citric acid cycle. The process releases some energy but more importantly paves the way for vast amounts of energy to be made through the citric acid cycle. After glucose has been phosphorylated and isomerized to Fructose-6-phospate, PFK begins its work. It phosphorylates the hydroxy group at the number one carbon, which was impossible in glucose. This second phosphorylation by PFK is important because it makes a doubly-high-energy compound. High energy compounds, like Fructose-6-phosphate and Fructose-1,6-bisphosphate, help to drive the endergonic processes of glycolysis through their own exergonic breakdown.[1]. It is important then that PFK makes a bisphosphate compound, because eventually that molecule will be cut in half. Thus, after the action of PFK, the six-carbon compound can be broken into two high-energy three-carbon compounds, which are both ready to move onto the next steps of glycolysis.
Structure
PFK is a that acts as a dimer of dimers, similar to hemoglobin.[2]. One half of each dimer is involved in the binding of ATP, while the other is involved with substrate binding and also contains an allosteric site. [3]. One subunit with ATP and F6P bound can be seen below. PFK's puts it in an alpha and beta class. Each unit is comprised of two domains that sandwich parallel beta sheets in between alpha helices. The outer most beta sheets of the larger domain, however, are anti-parallel. This is best seen in the .
Mechanism
Phosphofructokinase binds both Mg2+-ATP and fructose-6-phosphate (F6P) to make fructose-1,6-bisphosphate and Mg2+-ADP. Although the image with both of these products has not been determined, bound to the enzyme has been. There are three ligand binding sites per subunit. Two make up the active site, which binds F6P and ATP, while the third is an allosteric binding site.[4] Some proposed residues involved at the active site include .[5] PFK exist in two conformational states, both and which are in equilibrium. ATP binds both active and allosteric sites in both conformations.
Regulation
Glycolysis is an essential metabolic process for survival. Therefore, in its activation and suppression it must be highly regulated. Three points in the process of glycolysis occur with a large negative free energy and are therefore, irreversible. These three points are hexokinase, phosphofructokinase, and pyruvate kinase. These three reactions are candidates to be the major points of regulation, because they are they committed steps. Of the three PFK is considered the major regulatory point for glycolysis in muscle with a ΔG= -25.9 kJ/mol. [6]. This makes sense seeing as pyruvate kinase catalyzes the final reaction and hexokinase is an enzyme involved in more processes than glycolysis.
PFK is regulated by ATP, AMP, ADP. While ATP binds at the active site equally well in both R and T states, it preferentially binds the allosteric site of the T state [7] This preferential binding causes a shift from equilibrium of the two states, to a greater amount of T state [8], which decreases the affinity for F6P. Allosteric activator also binds to allosteric site to increase the ratio of R state phosphofructokinase.
The system of regulation matches perfectly with the function of PFK. When PFK is active, ATP is being produced down stream from it as glucose is eventually broken down completely. Thus, when ATP levels are low and more needs to be made, the activity of PFK will be increased, because ADP will be in high concentration. The opposite holds true as well, when ATP is in high concentration and inhibits protein activity.
The PFK's Km for ATP is .020mM and .032mM.[9]
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Evans PR, Farrants GW, Hudson PJ. Phosphofructokinase: structure and control. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):53-62. PMID:6115424
- ↑ Shirakihara Y, Evans PR. Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products. J Mol Biol. 1988 Dec 20;204(4):973-94. PMID:2975709
- ↑ Evans PR, Farrants GW, Hudson PJ. Phosphofructokinase: structure and control. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):53-62. PMID:6115424
- ↑ http://www.nature.com/nature/journal/v327/n6121/abs/327437a0.html
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ PubMed:2136935
- ↑ Campos G, Guixe V, Babul J. Kinetic mechanism of phosphofructokinase-2 from Escherichia coli. A mutant enzyme with a different mechanism. J Biol Chem. 1984 May 25;259(10):6147-52. PMID:6233271