Sandbox Reserved 194
From Proteopedia
| Line 14: | Line 14: | ||
[[Image:1RTAnew.png|thumb|left|275px|Thymidylic acid tetramer complexed with ribonuclease A]] | [[Image:1RTAnew.png|thumb|left|275px|Thymidylic acid tetramer complexed with ribonuclease A]] | ||
| + | == Substrate Binding == | ||
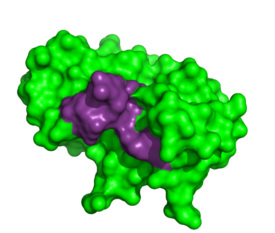
To determine the structural characteristics of RNA substrate binding to RNase A, X-ray crystallography was used to image inhibitory DNA tetramers bound to the enzyme. DNA lacks the 2′OH essential to RNA cleavage, making the complex more conducive to crystallography. In previous studies, <scene name='Sandbox_Reserved_194/1rta_structure/1'> | To determine the structural characteristics of RNA substrate binding to RNase A, X-ray crystallography was used to image inhibitory DNA tetramers bound to the enzyme. DNA lacks the 2′OH essential to RNA cleavage, making the complex more conducive to crystallography. In previous studies, <scene name='Sandbox_Reserved_194/1rta_structure/1'> | ||
| Line 25: | Line 26: | ||
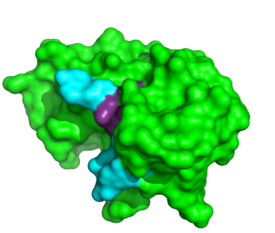
for adenosine bases at these two positions. RNase A establishes <scene name='Sandbox_Reserved_194/1rcn_his/9'>pi stacking between His119 and A3 </scene>in addition to hydrogen bonding between <scene name='Sandbox_Reserved_194/1rcn_hydrogen_bonding/9'>Asn71-A3, Gln69-A3 and Gln69-A4</scene>. ‘<ref>PMID:8063789</ref>’ | for adenosine bases at these two positions. RNase A establishes <scene name='Sandbox_Reserved_194/1rcn_his/9'>pi stacking between His119 and A3 </scene>in addition to hydrogen bonding between <scene name='Sandbox_Reserved_194/1rcn_hydrogen_bonding/9'>Asn71-A3, Gln69-A3 and Gln69-A4</scene>. ‘<ref>PMID:8063789</ref>’ | ||
| + | == Conclusion == | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 01:01, 15 April 2011
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
Contents |
Ribonuclease A Substrate Binding
Introduction
|
RNA degradation and formation is essential in protein regulation within the body. RNase A is an endonuclease that cleaves and breaks down RNA through attack at its 2’OH. It has served as a model providing insight into the stability, folding and chemistry of proteins. ‘[1]’ ‘[2]’
Substrate Binding
To determine the structural characteristics of RNA substrate binding to RNase A, X-ray crystallography was used to image inhibitory DNA tetramers bound to the enzyme. DNA lacks the 2′OH essential to RNA cleavage, making the complex more conducive to crystallography. In previous studies, has provided information into the specificity of the binding pocket subunits, B0, B1, B2 and B3. Many interactions observed in this complex occur between amino acid residues and the nucleic acid backbone such as hydrogen bonding between the . Because of the interactions with the , it appears this site is exclusive to pyrimidines. ‘[3]’
Further binding pocket characterization was accomplished using the oglionucleotide .
While binding of other nucleobases to the B2 and B3 sites is possible, the imaging of this elucidated the preferences
for adenosine bases at these two positions. RNase A establishes in addition to hydrogen bonding between . ‘[4]’
Conclusion
References
- ↑ Raines RT. Ribonuclease A. Chem Rev. 1998 May 7;98(3):1045-1066. PMID:11848924
- ↑ Leonidas, Demetres. "Ribonuclease A." 1997.
- ↑ Birdsall DL, McPherson A. Crystal structure disposition of thymidylic acid tetramer in complex with ribonuclease A. J Biol Chem. 1992 Nov 5;267(31):22230-6. PMID:1429575
- ↑ Fontecilla-Camps JC, de Llorens R, le Du MH, Cuchillo CM. Crystal structure of ribonuclease A.d(ApTpApApG) complex. Direct evidence for extended substrate recognition. J Biol Chem. 1994 Aug 26;269(34):21526-31. PMID:8063789