This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox89220
From Proteopedia
| Line 18: | Line 18: | ||
PMM2 converts mannose-6-phosphate into mannose-1-phosphate that would then serve as the building bloc of the dolichol-linked oligosaccharides for glycosylation in endoplasmic reticulum. It's not clear how PMM2 the cap and core domains would coordinate with each other. However,through the extrapolation of its paralogous protein, PMM1 which has a complex mechanism that uses charges of the conserved residues in the cap domains to sweep the substrate into core domain, we could postulate that PMM2 would utilize similar machinery. Also, with the close resemblance to the PGM proteins, PMM2 might have similar machinery that manipulate the conformational changes that “closes” cap domain after binding substrates to allow the specificity loops getting into contact with the active site of the core domain, thus participating in catalysis. | PMM2 converts mannose-6-phosphate into mannose-1-phosphate that would then serve as the building bloc of the dolichol-linked oligosaccharides for glycosylation in endoplasmic reticulum. It's not clear how PMM2 the cap and core domains would coordinate with each other. However,through the extrapolation of its paralogous protein, PMM1 which has a complex mechanism that uses charges of the conserved residues in the cap domains to sweep the substrate into core domain, we could postulate that PMM2 would utilize similar machinery. Also, with the close resemblance to the PGM proteins, PMM2 might have similar machinery that manipulate the conformational changes that “closes” cap domain after binding substrates to allow the specificity loops getting into contact with the active site of the core domain, thus participating in catalysis. | ||
| + | |||
| + | ---- | ||
| + | ==Evolutionary History== | ||
| + | |||
| + | Both PMM1 and PMM2 are grouped under the haloacid dehydrogenase family, which has 4 characteristic motifs that are highly conserved in both PMMs. The phylegenic analysis of PMMs in 26 species of animals reveals a good complementary data to explain the divergence of the functions between these so closely related proteins in humans. Apparently, in the early stages of evolution of vertebrates or before the divergence of vertebrates, there are the duplication events of the PMM gene in the common vertebrates’ ancestor. The duplication allows extra set of genomic information to be passed on and mutations to occur, which later leads to the emergence of the similar yet distinct PMM1 and PMM2. Judging from the higher degree of identity between certain yeast type and human’s PMM2,it's said that PMM2 evolves more slowly than PMM1. However, since mutations can occur on both set of genomic information in the duplication events, hence, it couldn’t be deduced that if the evolutionary rate of the PMMs have any associations with the mutations on the duplicated portion of the gene. As a matter of fact, with the different evolutionary rates after duplication, it allows the PMM1 and PMM2 to have diverged functions in their active sites which have some degree of conservations throughout the evolutionary lineage. It doesn't escape the attention that there are different residues replacement in primary and secondary specificity loop in the highly conserved motifs 1 and 2 in both proteins. For example, while PMM1 can be stimulated by IMP in the brain to increase its phosphatase activity (as glucose-1,6- bisphosphatase) and has decreased activity as phosphomutase by then, IMP is shown to has no effect on both the phosphatase and phosphomutase activity of PMM2. Again, it’s obvious that the evolutionary development of the PMM genes after duplication results in these two similar yet distinct proteins. | ||
Revision as of 04:15, 19 April 2011
Contents |
Phosphomannose mutase 2
Background
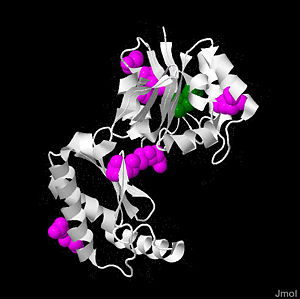
Structure
|
PMM2 is a 246 amino acid long protein, with 2 identical subunits working as independent domains, hence it’s also known as a homodimeric protein. In addition, it’s classified as the member of HAD (haloacid dehydrolase) superfamily due to the fact that it had 4 different types of motifs, each of which is highly conserved across the vertebrates’ lineages during evolutions. Similar to other members in HAD superfamily, the two domains are separately recognized as core and cap domain. The core domain (residues 1 – 90, and 198-262)of PMM2 displays the characteristic 4 motifs of HAD superfamily that contribute to the catalytic ability of the active sites in the protein. Motif 1 has Asp as nucleophile which serves as the mediator for the phosphoryl group transfer, the second Asp, on the other hand, acts as in the general acid-base reaction. While motif 1 provides the nucleophilic and general acid-base reactions, motif 2 in the protein which usually contains Thr or Ser helps in positioning or binding on the substrates’ phosphoryl group. In addition, motif 3 has the typical Lys or Arg residues while motif 4 has the acidic residues (Asp and Glu) that bind magnesium cofactor. The cap domain of PMM2 is smaller, functioning as the regulator for the access of the substrate into the active site of the core domain and at the same time contains the primary and secondary substrate specificity loops that recognize only specific substrate.
How does PMM2 work?
PMM2 converts mannose-6-phosphate into mannose-1-phosphate that would then serve as the building bloc of the dolichol-linked oligosaccharides for glycosylation in endoplasmic reticulum. It's not clear how PMM2 the cap and core domains would coordinate with each other. However,through the extrapolation of its paralogous protein, PMM1 which has a complex mechanism that uses charges of the conserved residues in the cap domains to sweep the substrate into core domain, we could postulate that PMM2 would utilize similar machinery. Also, with the close resemblance to the PGM proteins, PMM2 might have similar machinery that manipulate the conformational changes that “closes” cap domain after binding substrates to allow the specificity loops getting into contact with the active site of the core domain, thus participating in catalysis.
Evolutionary History
Both PMM1 and PMM2 are grouped under the haloacid dehydrogenase family, which has 4 characteristic motifs that are highly conserved in both PMMs. The phylegenic analysis of PMMs in 26 species of animals reveals a good complementary data to explain the divergence of the functions between these so closely related proteins in humans. Apparently, in the early stages of evolution of vertebrates or before the divergence of vertebrates, there are the duplication events of the PMM gene in the common vertebrates’ ancestor. The duplication allows extra set of genomic information to be passed on and mutations to occur, which later leads to the emergence of the similar yet distinct PMM1 and PMM2. Judging from the higher degree of identity between certain yeast type and human’s PMM2,it's said that PMM2 evolves more slowly than PMM1. However, since mutations can occur on both set of genomic information in the duplication events, hence, it couldn’t be deduced that if the evolutionary rate of the PMMs have any associations with the mutations on the duplicated portion of the gene. As a matter of fact, with the different evolutionary rates after duplication, it allows the PMM1 and PMM2 to have diverged functions in their active sites which have some degree of conservations throughout the evolutionary lineage. It doesn't escape the attention that there are different residues replacement in primary and secondary specificity loop in the highly conserved motifs 1 and 2 in both proteins. For example, while PMM1 can be stimulated by IMP in the brain to increase its phosphatase activity (as glucose-1,6- bisphosphatase) and has decreased activity as phosphomutase by then, IMP is shown to has no effect on both the phosphatase and phosphomutase activity of PMM2. Again, it’s obvious that the evolutionary development of the PMM genes after duplication results in these two similar yet distinct proteins.
