User:Momodou L. Jammeh/Sandbox 1
From Proteopedia
| Line 5: | Line 5: | ||
===INTRODUCTION=== | ===INTRODUCTION=== | ||
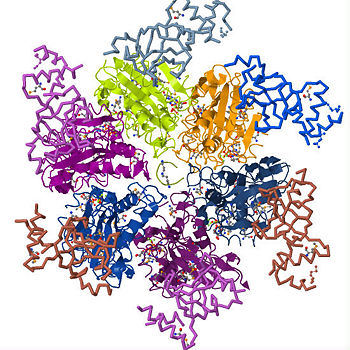
| - | Transcription is the process of mRNA synthesis by RNA polymerase, an enzyme that uses a strand of DNA as a template for ribonucloetide addition. During this process, RNA polymerase encounters several regulatory proteins, called transcription factors, that effect transcription in various ways. One of the major regulatory activities of transcription regulators is terminating the process at specific sites in the transcription unit. In ''E. coli'' and prokaryotes in general, transcription is terminated through rho dependent and independent mechanisms. Transcription termination without rho occurs through RNA polymerase's intrinsic ability to terminate transcription in response to certain, limited sequences in the template DNA strand. Rho dependent termination requires a helicase protein called rho factor which belongs in the helicase class of enzymes. Helicases are motor proteins that function through directional movement on the nucleic acid phosphodiester backbone and can dissociate bound DNA-DNA, RNA-DNA or RNA-RNA strands. Rho specifically terminates transcription by separating the the new RNA strand annealed to the DNA strand being transcribed. As pictured on the upper right corner, it is a hexameric ring-shaped helicase that uses energy derived from its ATPase mechanism (hydrolysis of ATP into ADP and an organic phosphate) to drive its movement along the newly formed RNA molecule toward the elongation complex to be dissociated. While rho is only requirement for the termination process, additional elongation factors such as NusA and NusG have been shown to enhance the process. NusA reduces the elongation rate allowing rho to catch up with RNA polymerase despite its high processivity while NusG enhances the rate of RNA release by coupling rho to the elongation complex. Since its discovery in 1969, studies on rho have been essential in understanding | + | Transcription is the process of mRNA synthesis by RNA polymerase, an enzyme that uses a strand of DNA as a template for ribonucloetide addition. During this process, RNA polymerase encounters several regulatory proteins, called transcription factors, that effect transcription in various ways. One of the major regulatory activities of transcription regulators is terminating the process at specific sites in the transcription unit. In ''E. coli'' and prokaryotes in general, transcription is terminated through rho dependent and independent mechanisms. Transcription termination without rho occurs through RNA polymerase's intrinsic ability to terminate transcription in response to certain, limited sequences in the template DNA strand. Rho dependent termination requires a helicase protein called rho factor which belongs in the helicase class of enzymes. Helicases are motor proteins that function through directional movement on the nucleic acid phosphodiester backbone and can dissociate bound DNA-DNA, RNA-DNA or RNA-RNA strands. Rho specifically terminates transcription by separating the the new RNA strand annealed to the DNA strand being transcribed. As pictured on the upper right corner, it is a hexameric ring-shaped helicase that uses energy derived from its ATPase mechanism (hydrolysis of ATP into ADP and an organic phosphate) to drive its movement along the newly formed RNA molecule toward the elongation complex to be dissociated. While rho is only requirement for the termination process, additional elongation factors such as NusA and NusG have been shown to enhance the process. NusA reduces the elongation rate allowing rho to catch up with RNA polymerase despite its high processivity while NusG enhances the rate of RNA release by coupling rho to the elongation complex. Pause signal sequences are also common at rho termination sites at the elongation complex is halted at these sequences making it more susceptible to rho termination. Since its discovery in 1969, studies on rho have been essential in understanding how external factors regulation transcription termination and its structure provides insight into how several stable hexameric helicases load onto their nucleic acid substrates. |
---- | ---- | ||
| Line 26: | Line 26: | ||
[[Image:rho.jpg|350px||left|thumb|Fig. 2 The diagram shows nascent RNA (dashes) emerging from the exit site of RNA polymerase and zig-zagging though the six primary RNA binging sites of rho. Taken from Richardson, JP (2003)]] | [[Image:rho.jpg|350px||left|thumb|Fig. 2 The diagram shows nascent RNA (dashes) emerging from the exit site of RNA polymerase and zig-zagging though the six primary RNA binging sites of rho. Taken from Richardson, JP (2003)]] | ||
===MECHANISM=== | ===MECHANISM=== | ||
| - | Highlights of <scene name='User:Momodou_L._Jammeh/Sandbox_1/Rho_catatylc_site/1'>RHO</scene>'s functional sub-domains. The termination of transcription by rho requires the binding of nascent RNA by the hexamer which functions as an RNA-activated ATPase helicase. This molecular motor is driven by ATP hydrolysis which occurs in the main catalytic site in the C-terminal domain of rho monomers. Rho makes contact with its substrate, nascent RNA transcript, through its OB fold which serves as the primary RNA binding site. The OB fold fold recognizes and binds to the cytosine-rich region of a transcript called the ''r''ho ''ut''ilization (''rut'') sequence of 40 or more bases. Thus, the N-terminus domain makes first contact with RNA and the loading process begins. Once the OB fold makes contact with the mRNA, a notch forms in rho turning it into an open ring and allows the mRNA to load unto the protein and associate with the Q and R loops of the secondary binding site. The first cytidine base fits tightly within a hydrophobic pocket formed by Phe62 and Tyr80, and it also contacts residues Glu108, Arg109, and Tyr110 in the RNA-binding domain. The second cytidine forms specific hydrogen bonds with Arg66 and Asp78 and stacks on the aromatic side chain of Phe64. This stimulates ring closer and also alters the conformation of the P-loop such that it is appropriate for ATP binding and subsequent hydrolysis, an ATPase. Although rho has six potential ATPase sites, it functions as a trimer of dimers through sequential firing of three of the ATPase sites. Rho's ATPase activity is stimulated by the mRNA whose fourth nucloetide from the 5' end is bound by Lys181 and orients it toward Lys184 which links the ATPase active site to the RNA substrate. The energy derived from ATP hydrolysis drives the translocation of rho as it moves along the nucleic acid track. | + | Highlights of <scene name='User:Momodou_L._Jammeh/Sandbox_1/Rho_catatylc_site/1'>RHO</scene>'s functional sub-domains. The termination of transcription by rho requires the binding of nascent RNA by the hexamer which functions as an RNA-activated ATPase helicase. This molecular motor is driven by ATP hydrolysis which occurs in the main catalytic site in the C-terminal domain of rho monomers. Rho makes contact with its substrate, nascent RNA transcript, through its OB fold which serves as the primary RNA binding site. The OB fold fold recognizes and binds to the cytosine-rich region of a transcript called the ''r''ho ''ut''ilization (''rut'') sequence of 40 or more bases. Thus, the N-terminus domain makes first contact with RNA and the loading process begins. Once the OB fold makes contact with the mRNA, a notch forms in rho turning it into an open ring and allows the mRNA to load unto the protein and associate with the Q and R loops of the secondary binding site. The first cytidine base fits tightly within a hydrophobic pocket formed by Phe62 and Tyr80, and it also contacts residues Glu108, Arg109, and Tyr110 in the RNA-binding domain. The second cytidine forms specific hydrogen bonds with Arg66 and Asp78 and stacks on the aromatic side chain of Phe64. This stimulates ring closer and also alters the conformation of the P-loop such that it is appropriate for ATP binding and subsequent hydrolysis, an ATPase. Although rho has six potential ATPase sites, it functions as a trimer of dimers through sequential firing of three of the ATPase sites. Rho's ATPase activity is stimulated by the mRNA whose fourth nucloetide from the 5' end is bound by Lys181 and orients it toward Lys184 which links the ATPase active site to the RNA substrate. The energy derived from ATP hydrolysis drives the translocation of rho as it moves along the nucleic acid track. This second step in rho dependent termination occurs in 5' to 3' fashion as the RNA threads through the central cavity of rho. In addition, the RNA also wraps around the top part of the low affinity c-terminal residues of rho as it moves toward the RNA polymer-DNA complex which it eventually dissociates with forces from ATP hydrolysis. |
---- | ---- | ||
===INHIBITION=== | ===INHIBITION=== | ||
---- | ---- | ||
Revision as of 13:56, 29 April 2011
Contents |
RHO TERMINATION FACTOR
INTRODUCTION
Transcription is the process of mRNA synthesis by RNA polymerase, an enzyme that uses a strand of DNA as a template for ribonucloetide addition. During this process, RNA polymerase encounters several regulatory proteins, called transcription factors, that effect transcription in various ways. One of the major regulatory activities of transcription regulators is terminating the process at specific sites in the transcription unit. In E. coli and prokaryotes in general, transcription is terminated through rho dependent and independent mechanisms. Transcription termination without rho occurs through RNA polymerase's intrinsic ability to terminate transcription in response to certain, limited sequences in the template DNA strand. Rho dependent termination requires a helicase protein called rho factor which belongs in the helicase class of enzymes. Helicases are motor proteins that function through directional movement on the nucleic acid phosphodiester backbone and can dissociate bound DNA-DNA, RNA-DNA or RNA-RNA strands. Rho specifically terminates transcription by separating the the new RNA strand annealed to the DNA strand being transcribed. As pictured on the upper right corner, it is a hexameric ring-shaped helicase that uses energy derived from its ATPase mechanism (hydrolysis of ATP into ADP and an organic phosphate) to drive its movement along the newly formed RNA molecule toward the elongation complex to be dissociated. While rho is only requirement for the termination process, additional elongation factors such as NusA and NusG have been shown to enhance the process. NusA reduces the elongation rate allowing rho to catch up with RNA polymerase despite its high processivity while NusG enhances the rate of RNA release by coupling rho to the elongation complex. Pause signal sequences are also common at rho termination sites at the elongation complex is halted at these sequences making it more susceptible to rho termination. Since its discovery in 1969, studies on rho have been essential in understanding how external factors regulation transcription termination and its structure provides insight into how several stable hexameric helicases load onto their nucleic acid substrates.
STRUCTURE
|
MECHANISM
Highlights of 's functional sub-domains. The termination of transcription by rho requires the binding of nascent RNA by the hexamer which functions as an RNA-activated ATPase helicase. This molecular motor is driven by ATP hydrolysis which occurs in the main catalytic site in the C-terminal domain of rho monomers. Rho makes contact with its substrate, nascent RNA transcript, through its OB fold which serves as the primary RNA binding site. The OB fold fold recognizes and binds to the cytosine-rich region of a transcript called the rho utilization (rut) sequence of 40 or more bases. Thus, the N-terminus domain makes first contact with RNA and the loading process begins. Once the OB fold makes contact with the mRNA, a notch forms in rho turning it into an open ring and allows the mRNA to load unto the protein and associate with the Q and R loops of the secondary binding site. The first cytidine base fits tightly within a hydrophobic pocket formed by Phe62 and Tyr80, and it also contacts residues Glu108, Arg109, and Tyr110 in the RNA-binding domain. The second cytidine forms specific hydrogen bonds with Arg66 and Asp78 and stacks on the aromatic side chain of Phe64. This stimulates ring closer and also alters the conformation of the P-loop such that it is appropriate for ATP binding and subsequent hydrolysis, an ATPase. Although rho has six potential ATPase sites, it functions as a trimer of dimers through sequential firing of three of the ATPase sites. Rho's ATPase activity is stimulated by the mRNA whose fourth nucloetide from the 5' end is bound by Lys181 and orients it toward Lys184 which links the ATPase active site to the RNA substrate. The energy derived from ATP hydrolysis drives the translocation of rho as it moves along the nucleic acid track. This second step in rho dependent termination occurs in 5' to 3' fashion as the RNA threads through the central cavity of rho. In addition, the RNA also wraps around the top part of the low affinity c-terminal residues of rho as it moves toward the RNA polymer-DNA complex which it eventually dissociates with forces from ATP hydrolysis.