User:Caitlin Bell/Sandbox 1
From Proteopedia
| Line 25: | Line 25: | ||
Third, once the stimulatory G protein is bound to an ADP-ribose, it is permanently active. This means that it is unable to hydrolyze its GTP, which would normally allow the protein to “switch off”. Thus, the stimulatory G protein continually activates adenylyl cyclase, which leads to a constant production of cAMP. Consequently, cAMP activates Protein Kinase A, which activates the CFTR-regulated Cl- channel. Constant activation of this ion channel results in an enormous efflux of ions and water from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera (4). | Third, once the stimulatory G protein is bound to an ADP-ribose, it is permanently active. This means that it is unable to hydrolyze its GTP, which would normally allow the protein to “switch off”. Thus, the stimulatory G protein continually activates adenylyl cyclase, which leads to a constant production of cAMP. Consequently, cAMP activates Protein Kinase A, which activates the CFTR-regulated Cl- channel. Constant activation of this ion channel results in an enormous efflux of ions and water from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera (4). | ||
| + | |||
| + | After the victim has ingested Vibrio cholerea, it travels from the oral cavity to the intestines, where it secretes the cholera toxin within the mucus lining. In the intestines, the B subunit pentamer binds to the lining of the intestinal wall through its five <scene name='User:Caitlin_Bell/Sandbox_1/Gm1_binding_sites/3'>GM1 binding site</scene>. Once bound, the toxin is engulfed into the cell through endocytosis, and immediately following, the A1 domain is proteolytically cleaved from A2 between residues 182 and 184. Before the A1 chain is considered fully active, the Cys187-Cys199 disulfide bond is reduced, which takes place 20A from the active site of A1. Consequently, A1 is free from the rest of the protein and is able to perform its enzymatic duties. | ||
| + | |||
| + | In order for the ADP-ribosylation reaction to occur, the binding affinity of NAD+ must be increased. This is accomplished through a set of ADP-ribosylation factors (ARFs) that are bound with GTP and are found within the cells. The ARFs bind 15A from the catalytic site and allow the A1 chain to bind NAD+ through its cluster of Arg residues at the <scene name='User:Caitlin_Bell/Sandbox_1/Catalytic_site/2'>catalytic binding site</scene>, which play an important role in stabilizing the binding through multiple van der waals interactions and hydrogen bonds. However, these Arg residues do not play a catalytic role. The <scene name='User:Caitlin_Bell/Sandbox_1/Glu_res/1'>Glutamine 112 residue</scene> residue of the A1 chain is the essential component in removing the ADP-ribose from NAD+, which is then transferred to the Arg201 residue on the alpha subunit of a stimulatory G protein. | ||
| + | |||
| + | From this ADP-ribosylation reaction, the stimulatory G protein remains in its active state, which means that it is permanently bound to GTP. Thus begins the second messenger downstream events. The active G protein continually activates adenylyl cyclase, which constantly produces the cAMP messenger. The cAMP messenger activates Protein Kinase A, which then uses the energy of hydrolysis of ATP to activate the CFTR-Cl- channel. Constant activation of this ion channel leads to the subsequent massive efflux of water and electrolytes from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera. Constant activation of the CFTR-Cl- channel also results in severe metabolic acidosis (Bhagavan 2011). Because the attached ADP-ribose makes the G protein unable to deactivate, this cycle is continuous. | ||
----- | ----- | ||
Revision as of 18:18, 5 May 2011
Contents |
CHOLERA TOXIN
Introduction
The cholera toxin is released by the pathogen Vibrio cholerae during colonization of the small intestine. Vibrio organisms are mainly found in saltwater, but there some that live in freshwater. These organisms use glucose for energy, and they use flagella for locomotion.
Cholera is widespread in mainly poverty-stricken areas where food and water environments are unsanitary. After ingestion of Vibrio cholerae, which typically is a result of feces particles in water or food, the cholera toxin is secreted and infects the small intestines, leading to the Cholera disease. Excessive diarrhea and vomiting ensues soon after ingestion, and death can occur within a few hours. Cholera is prevalent in Africa, Asia, and Latin America, leading to 3-5 million cases and 100,000-200,000 deaths every year (1).
|
Structure
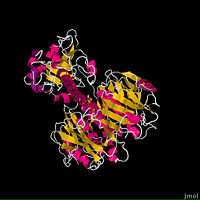
The fundamental structure of the cholera toxin is rather basic. It is a complex of six proteins that are structured into two subunits: A and B. The A subunit contains only one protein and is the only toxic part of the protein. The B subunit contains five proteins and is non-toxic.
The is the most destructive part of the cholera toxin. It can be further broken down into two chains: A1 and A2. These chains are held together through a peptide bond and single disulfide bond. The is entirely non-polar, which allows it to pass through the intestinal membrane and into the cell. The A1 chain has a for NAD+, and when bound, begins the downstream events that ultimately leads to the Cholera disease. The 's main function is to connect the A1 chain to the B subunit through the B subunit's cylindric pore (2).
The ’s only function is to allow the cholera toxin to enter the intestinal epithelial cells through endocytosis. It accomplishes this from its unique structure. The B subunit contains five alpha helix proteins that are connected together to form a pentagon. Each alpha helix contains a single binding site for the intestinal lining, which is called its (monosialoganglioside binding site) (2).
Mechanism of Action
The mechanism of action of the cholera toxin can be broken down into three simple stages: entry of the toxin into the cell, activation of the G protein through its catalytic functions, and efflux of ions.
First, after ingestion of Vibrio cholerae, the cholera toxin is secreted in the intestines. Once inside, the B subunit binds to the intestinal wall through its . The cholera toxin is then engulfed by the intestinal epithelial cell through endocytosis, and immediately following, the A subunit is cleaved at the peptide bond that connects the A1 chain and A2 chain. To completely activate the A1 chain, a disulfide bond is reduced.
Second, after the A1 chain is released inside the intestinal cell, it binds NAD+ at its . The affinity for this interaction is greatly increased with the binding of ADP-Ribosylation Factors (ARFs), which bind near the catalytic site of A1. Once bound, the removes an ADP-ribose from it, and the A1 chain proceeds to transfer this to a stimulatory G protein. This transfer is called an ADP-ribosylation reaction (3).
Third, once the stimulatory G protein is bound to an ADP-ribose, it is permanently active. This means that it is unable to hydrolyze its GTP, which would normally allow the protein to “switch off”. Thus, the stimulatory G protein continually activates adenylyl cyclase, which leads to a constant production of cAMP. Consequently, cAMP activates Protein Kinase A, which activates the CFTR-regulated Cl- channel. Constant activation of this ion channel results in an enormous efflux of ions and water from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera (4).
After the victim has ingested Vibrio cholerea, it travels from the oral cavity to the intestines, where it secretes the cholera toxin within the mucus lining. In the intestines, the B subunit pentamer binds to the lining of the intestinal wall through its five . Once bound, the toxin is engulfed into the cell through endocytosis, and immediately following, the A1 domain is proteolytically cleaved from A2 between residues 182 and 184. Before the A1 chain is considered fully active, the Cys187-Cys199 disulfide bond is reduced, which takes place 20A from the active site of A1. Consequently, A1 is free from the rest of the protein and is able to perform its enzymatic duties.
In order for the ADP-ribosylation reaction to occur, the binding affinity of NAD+ must be increased. This is accomplished through a set of ADP-ribosylation factors (ARFs) that are bound with GTP and are found within the cells. The ARFs bind 15A from the catalytic site and allow the A1 chain to bind NAD+ through its cluster of Arg residues at the , which play an important role in stabilizing the binding through multiple van der waals interactions and hydrogen bonds. However, these Arg residues do not play a catalytic role. The residue of the A1 chain is the essential component in removing the ADP-ribose from NAD+, which is then transferred to the Arg201 residue on the alpha subunit of a stimulatory G protein.
From this ADP-ribosylation reaction, the stimulatory G protein remains in its active state, which means that it is permanently bound to GTP. Thus begins the second messenger downstream events. The active G protein continually activates adenylyl cyclase, which constantly produces the cAMP messenger. The cAMP messenger activates Protein Kinase A, which then uses the energy of hydrolysis of ATP to activate the CFTR-Cl- channel. Constant activation of this ion channel leads to the subsequent massive efflux of water and electrolytes from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera. Constant activation of the CFTR-Cl- channel also results in severe metabolic acidosis (Bhagavan 2011). Because the attached ADP-ribose makes the G protein unable to deactivate, this cycle is continuous.
References
(1) “Cholera”. World Health Organization. June 2010. <http://www.who.int/mediacentre/factsheets/fs107/en/>.
(2) Zhang, Rong-Guang. “The Three-dimensional Crystal Structure of Cholera Toxin”. JMol Biology. Pages 563-573. 1995.
(3) O’Neal, Claire. “Structural Basis for Activation of Cholera Toxin by Human ARF6 GTP”. Science. Volume 309, pages 1093-1096. 12 August 2005.
(4) Bhagavan, NV. “Gastrointestinal Digestion and Absorption”. Essentials of Medical Biochemistry with Clinical Cases. Copyright 2011. Macmillan Company.