CAMP Dependent Protein Kinase, Catalytic Subunit
From Proteopedia
| Line 9: | Line 9: | ||
[[Image:PKA1.svg.png]] | [[Image:PKA1.svg.png]] | ||
<Structure load='2qcs' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='2qcs' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
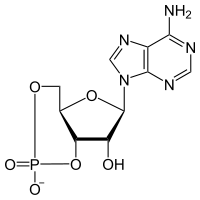
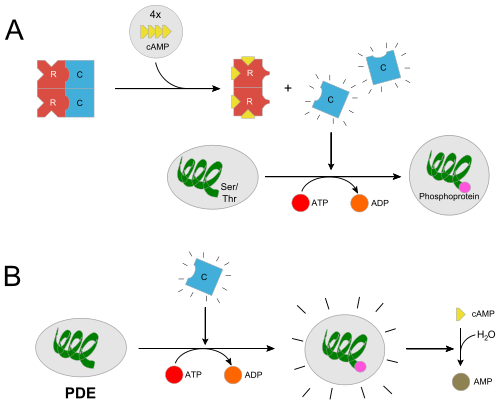
| - | In the inactive state, the catalytic subunit of PKA exists as a heterotetramer with two regulatory subunits and two catalytic subunits. Regulatory subunits often interact with A Kinase Anchoring proteins that serve to localize a population of PKA in a certain cellular environment, priming a particular response. Upon, cAMP binding to the regulatory domain of PKA (two molecules of cAMP per regulatory subunit) the catalytic subunit is released from the holoenzyme complex and is free to diffuse and exhibit its catalytic activity. Two of the most important residues for this docking interaction are <scene name='CAMP_Dependent_Protein_Kinase,_Catalytic_Subunit/Tyr247/1'>Tyr247</scene> | + | In the inactive state, the catalytic subunit of PKA exists as a heterotetramer with two regulatory subunits and two catalytic subunits. Regulatory subunits often interact with A Kinase Anchoring proteins that serve to localize a population of PKA in a certain cellular environment, priming a particular response. Upon, cAMP binding to the regulatory domain of PKA (two molecules of cAMP per regulatory subunit) the catalytic subunit is released from the holoenzyme complex and is free to diffuse and exhibit its catalytic activity. Two of the most important residues for this docking interaction are <scene name='CAMP_Dependent_Protein_Kinase,_Catalytic_Subunit/Tyr247/1'>Trp196</scene> and <scene name='CAMP_Dependent_Protein_Kinase,_Catalytic_Subunit/Tyr247/3'>Tyr247</scene> |
Revision as of 20:01, 6 May 2011
Introduction
The catalytic subunit of PKA is model kinase that is very well characterized because of its catalytic and structural simplicity and ease of Isolation. PKA is essential to cellular signalling in eukaryotes and is involved in a myriad of different processes depending on cell type. PKA catalyzes the transfer of a gamma-phosphoryl group of a molecule of ATP to either a serine or threonine residue on the target protein. This post-translational modification can serve to alter the function of the phosphorylated target protein.
Activation and Interaction with Regulatory Subunit
|
In the inactive state, the catalytic subunit of PKA exists as a heterotetramer with two regulatory subunits and two catalytic subunits. Regulatory subunits often interact with A Kinase Anchoring proteins that serve to localize a population of PKA in a certain cellular environment, priming a particular response. Upon, cAMP binding to the regulatory domain of PKA (two molecules of cAMP per regulatory subunit) the catalytic subunit is released from the holoenzyme complex and is free to diffuse and exhibit its catalytic activity. Two of the most important residues for this docking interaction are and