Antizyme Inhibitor
From Proteopedia
| Line 35: | Line 35: | ||
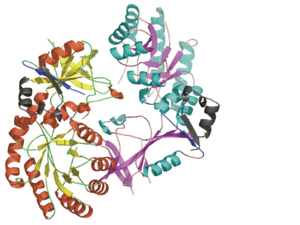
<scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. | <scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. | ||
</StructureSection> | </StructureSection> | ||
| + | |||
| + | ==3D structures of Antizyme Inhibitor== | ||
| + | |||
| + | [[3btn]] – AzI – mouse<br /> | ||
| + | [[1zo0]] – AzI – rat - NMR | ||
Revision as of 06:29, 9 May 2011
Crystal structure of the Antizyme inhibitor
| |||||||
| AzI, 3BTN | |||||||
|---|---|---|---|---|---|---|---|
| Coordinates: | save as pdb, mmCIF, xml | ||||||
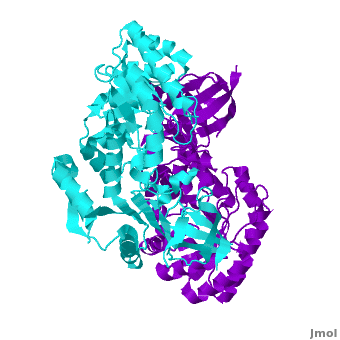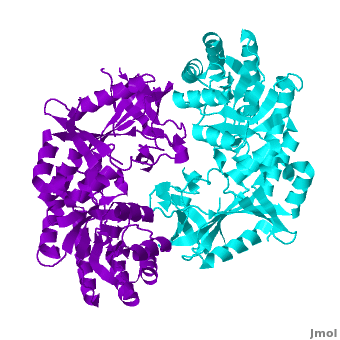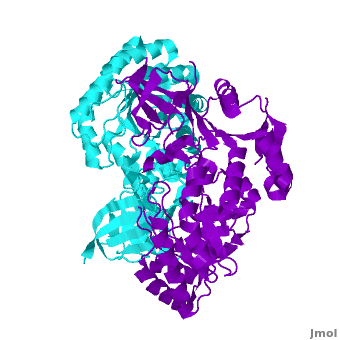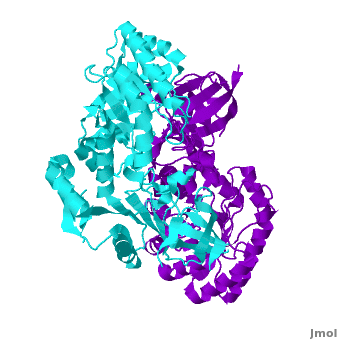
Antizyme inhibitor (AzI) regulates cellular polyamine homeostasis by binding to the polyamine-induced protein, Antizyme (Az), with greater affinity than ornithine decarboxylase (ODC). AzI is highly homologous to ODC but is not enzymatically active. In order to understand these specific characteristics of AzI and its differences from ODC, we determined the 3D structure of mouse AzI to 2.05 A resolution. Both AzI and ODC crystallize as a dimer. However, fewer interactions at the dimer interface, a smaller buried surface area, and lack of symmetry of the interactions between residues from the two monomers in the AzI structure suggest that this dimeric structure is nonphysiological. In addition, the absence of residues and interactions required for pyridoxal 5'-phosphate (PLP) binding suggests that AzI does not bind PLP. Biochemical studies confirmed the lack of PLP binding and revealed that AzI exists as a monomer in solution while ODC is dimeric. Our findings that AzI exists as a monomer and is unable to bind PLP provide two independent explanations for its lack of enzymatic activity and suggest the basis for its enhanced affinity toward Az.
Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function., Albeck S, Dym O, Unger T, Snapir Z, Bercovich Z, Kahana C, Protein Sci. 2008 May;17(5):793-802. Epub 2008 Mar 27. PMID:18369191
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
| |||||||||||
3D structures of Antizyme Inhibitor
3btn – AzI – mouse
1zo0 – AzI – rat - NMR
Additional Resources
For additional information, see: Cancer
Reference
Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Michal Harel, Joel L. Sussman, Dinesh Kulhary, David Canner, Jaime Prilusky, Orly Dym