We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
FOXP2
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
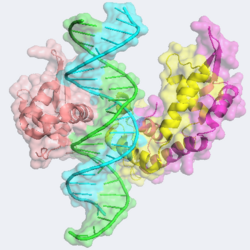
A very unique feature of the FOXP2-DNA structure is its ability to form a <scene name='FOXP2/Dimer_second/1'>domain swapped dimer</scene>. In the dimeric structure, two monomers exchange <scene name='FOXP2/Domain_swapped/1'>helix H3</scene>. The dimeric structure is stabilized by complex aromatic interactions involving <scene name='FOXP2/Aromatic/1'>aromatic residues</scene> 507, 509, 531, 534, 538, 540, 541, and 548. In typical FOX family proteins, dimerization is nearly impossible due to the presence of a proline at position 539, effectively preventing helix 2 and three from merging into a single long H3. In FOXP2, this proline is replaced with <scene name='FOXP2/Alanine/1'>an alanine</scene>, allowing the <scene name='FOXP2/Long_helices/1'>long helix</scene> to form. This feature is present in all FOXP proteins. Interestingly, the mutations Phe371Cys and Phe371Leu are known to cause FOXP dysfunction and speech issues, but <scene name='FOXP2/Buried_ph/1'>residue Phe 371 (Phe 538 here)</scene> is only involved in interactions in the FOXP2 dimeric form, highlighting the importance of the dimeric structure. The dimeric structure interacts with the DNA molecule to <scene name='FOXP2/Dimer_second_inter/2'>a far lesser extent</scene> than the monomeric form of FOXP2 however. A morph of the domain swapping can be <scene name='FOXP2/Domain_switching_morph/2'> seen here</scene>.<ref name="Chen"/> | A very unique feature of the FOXP2-DNA structure is its ability to form a <scene name='FOXP2/Dimer_second/1'>domain swapped dimer</scene>. In the dimeric structure, two monomers exchange <scene name='FOXP2/Domain_swapped/1'>helix H3</scene>. The dimeric structure is stabilized by complex aromatic interactions involving <scene name='FOXP2/Aromatic/1'>aromatic residues</scene> 507, 509, 531, 534, 538, 540, 541, and 548. In typical FOX family proteins, dimerization is nearly impossible due to the presence of a proline at position 539, effectively preventing helix 2 and three from merging into a single long H3. In FOXP2, this proline is replaced with <scene name='FOXP2/Alanine/1'>an alanine</scene>, allowing the <scene name='FOXP2/Long_helices/1'>long helix</scene> to form. This feature is present in all FOXP proteins. Interestingly, the mutations Phe371Cys and Phe371Leu are known to cause FOXP dysfunction and speech issues, but <scene name='FOXP2/Buried_ph/1'>residue Phe 371 (Phe 538 here)</scene> is only involved in interactions in the FOXP2 dimeric form, highlighting the importance of the dimeric structure. The dimeric structure interacts with the DNA molecule to <scene name='FOXP2/Dimer_second_inter/2'>a far lesser extent</scene> than the monomeric form of FOXP2 however. A morph of the domain swapping can be <scene name='FOXP2/Domain_switching_morph/2'> seen here</scene>.<ref name="Chen"/> | ||
</StructureSection> | </StructureSection> | ||
| + | |||
| + | ====Page Development==== | ||
| + | This article was developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University. | ||
| + | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 15:51, 17 May 2011
| |||||||||||
Page Development
This article was developed based on lectures given in Chemistry 543 by Prof. Clarence E. Schutt at Princeton University.
References
- ↑ Pinker S. Talk of genetics and vice versa. Nature. 2001 Oct 4;413(6855):465-6. PMID:11586336 doi:10.1038/35097173
- ↑ Enard W, Gehre S, Hammerschmidt K, Holter SM, Blass T, Somel M, Bruckner MK, Schreiweis C, Winter C, Sohr R, Becker L, Wiebe V, Nickel B, Giger T, Muller U, Groszer M, Adler T, Aguilar A, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Favor J, Fuchs H, Gailus-Durner V, Hans W, Holzlwimmer G, Javaheri A, Kalaydjiev S, Kallnik M, Kling E, Kunder S, Mossbrugger I, Naton B, Racz I, Rathkolb B, Rozman J, Schrewe A, Busch DH, Graw J, Ivandic B, Klingenspor M, Klopstock T, Ollert M, Quintanilla-Martinez L, Schulz H, Wolf E, Wurst W, Zimmer A, Fisher SE, Morgenstern R, Arendt T, de Angelis MH, Fischer J, Schwarz J, Paabo S. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009 May 29;137(5):961-71. PMID:19490899 doi:10.1016/j.cell.2009.03.041
- ↑ Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol. 2008 Sep;28(5):581-7. Epub 2008 May 15. PMID:18481161 doi:10.1007/s10875-008-9196-1
- ↑ 4.0 4.1 Stroud JC, Wu Y, Bates DL, Han A, Nowick K, Paabo S, Tong H, Chen L. Structure of the forkhead domain of FOXP2 bound to DNA. Structure. 2006 Jan;14(1):159-66. PMID:16407075 doi:10.1016/j.str.2005.10.005
See Also
- Forkhead box family
- Forkhead box protein M1
- Transcription & RNA Processing
- Regulation of Gene Expression
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Wayne Decatur, Alexander Berchansky