User:Bianca Varney/Bacterial Replication Termination
From Proteopedia
| Line 13: | Line 13: | ||
====RTP Structure==== | ====RTP Structure==== | ||
| - | Active RTP is a homodimer composed of 14.5 kDa subunits. The structure of the protein has been determined to a 2.6 A resolution using X-ray crystallography [3]. The RTP protein contains three major structural domains for its specific functionality; | + | Active RTP is a homodimer composed of 14.5 kDa subunits. The structure of the protein has been determined to a 2.6 A resolution using X-ray crystallography [3]. The RTP protein contains three major structural domains for its specific functionality; DNA-binding, DnaB interaction and dimer-dimer interaction domains [3]. The RTP is organized into a dimer by the association of their |
| + | <scene name='User:Bianca_Varney/Bacterial_Replication_Termination/Dimer_interaction_domain/1'>long α helices</scene> within the C-terminus [3]. The ‘<scene name='User:Bianca_Varney/Bacterial_Replication_Termination/Dna-binding/1'>winged helix</scene>’ is believed to be involved as the major DNA-binding domain while two α helices, found central in the protein, also fit adjacently into the major groove, and DNA-binding is a result of a three helical bundle [3]. This binding interaction is vastly different from the Tus-''ter'' interactions. | ||
====RTP Mechanism of Action==== | ====RTP Mechanism of Action==== | ||
| Line 19: | Line 20: | ||
An RTP dimer binds the core sequence and the complex formed allows a second dimer to cooperatively to bind to the auxiliary site. In the absence of a core site, the auxiliary site is unable to bind RTP. Furthermore, without the auxiliary site, RTP is unable to block the replication fork, as the interaction of both dimers has been suggested to provide enough DNA binding strength to displace the replication fork. This binding explains how the symmetrical RTP can block replication helicase machinery in an asymmetric manner. The blocking end occurs at the core site, while it is believed that the non-blocking auxiliary site may let replication through as there is less contact points of the dimer to the DNA and the replication machinery coming from this direction is predicted to displace the dimer that is weakly bound to the auxiliary site, which would then displace the dimer bound to the core [10]. Biochemical and mutational studies have identified particular residues that are vital for the functionality of RTP. Mutations within a hydrophobic region at residues Glu-30 and Tyr-33 causes the loss of contrahelicase ability [10]. These mutations do not affect dimer-dimer interactions or DNA binding activity and indicate that simple DNA binding is not able to block the replication fork. This provided evidence that RTP and the replication fork machinery interact specifically [10].</StructureSection> | An RTP dimer binds the core sequence and the complex formed allows a second dimer to cooperatively to bind to the auxiliary site. In the absence of a core site, the auxiliary site is unable to bind RTP. Furthermore, without the auxiliary site, RTP is unable to block the replication fork, as the interaction of both dimers has been suggested to provide enough DNA binding strength to displace the replication fork. This binding explains how the symmetrical RTP can block replication helicase machinery in an asymmetric manner. The blocking end occurs at the core site, while it is believed that the non-blocking auxiliary site may let replication through as there is less contact points of the dimer to the DNA and the replication machinery coming from this direction is predicted to displace the dimer that is weakly bound to the auxiliary site, which would then displace the dimer bound to the core [10]. Biochemical and mutational studies have identified particular residues that are vital for the functionality of RTP. Mutations within a hydrophobic region at residues Glu-30 and Tyr-33 causes the loss of contrahelicase ability [10]. These mutations do not affect dimer-dimer interactions or DNA binding activity and indicate that simple DNA binding is not able to block the replication fork. This provided evidence that RTP and the replication fork machinery interact specifically [10].</StructureSection> | ||
| - | ==The Terminus Utilization Substance ( | + | ==The Terminus Utilization Substance (''Escherichia coli'' )== |
<StructureSection load='2ewj' size='500' side='left' caption= 'Tus complexed to the ''E. coli'' ''ter'' site' scene=''>The ''E.coli'' protein that is responsible for termination is a 36kDa protein named Tus (Terminius Utilization Substance) that binds 23bp ''ter'' sites and arrests the replication helicase, DnaB, responsible for separating the two strands of DNA []. Unlike RTP termination sites, the ten ''E.coli'' ''ter'' sites do not contain inverted sequences or direct repeats and Tus binds as a monomer to a highly conserved core region of 13bp [8]. The tus-''ter'' complex is known to terminate replication by arresting the replication machinery in a in a polar manner however there is great discrepancy in evidence whether Tus specifically interacts or physically blocks the DnaB helicase to arrest its progression [1]. | <StructureSection load='2ewj' size='500' side='left' caption= 'Tus complexed to the ''E. coli'' ''ter'' site' scene=''>The ''E.coli'' protein that is responsible for termination is a 36kDa protein named Tus (Terminius Utilization Substance) that binds 23bp ''ter'' sites and arrests the replication helicase, DnaB, responsible for separating the two strands of DNA []. Unlike RTP termination sites, the ten ''E.coli'' ''ter'' sites do not contain inverted sequences or direct repeats and Tus binds as a monomer to a highly conserved core region of 13bp [8]. The tus-''ter'' complex is known to terminate replication by arresting the replication machinery in a in a polar manner however there is great discrepancy in evidence whether Tus specifically interacts or physically blocks the DnaB helicase to arrest its progression [1]. | ||
Revision as of 04:58, 21 May 2011
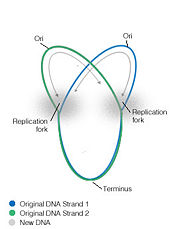
In most bacterial DNA replication initiation occurs at an origin where, due to the circular nature of the chromosome, the replication forks move bidirectionally to end at approxiametly 180 degrees away, at a specific sequence termini region [1]. Bacterial replication termination systems have been well studied in Eschericia coli and Bascillus subtilis. In both systems a trans-acting replication termination protein binds to a specific cis-acting DNA sequences, the replication termini (ter), and the DNA-protein complex arrests the progression of replication forks. The terminator sites are orientated so that protein binding is asymmetric, allowing the complexes to block the replication machinery from only one direction while letting them proceed unimpeded from the other direction [2]. In this way they are said to act in a polar manner. The proteins involved in this termination are non-homologous and differ structurally in E.coli and B.subtilis, although each contains similar contrahelicase activity and performs similar functions in arresting replication.
Contents |
Termination (ter) Sites
Replication is terminated in bacterial systems such as E.coli and B.subtilis by a "replication fork trap", studded with termination sites which causes the bidirectional forks to pause, encounter and fuse within a region called the terminus region. In E.coli the termination regions are spread across nearly half the chromosome compared to B.subtilis where they cover only ~10%. Termination regions are made up of two groups, opposite to each other, containing inverted sequences for the polar arrest of the replication helicase. In E.coli the 5 ter sites, J, G, F, B and C are arranged opposed to ter sites H, I, E, D and A, and can arrest the fork progressing in the clockwise direction and can block the anticlockwise direction, respectively. The replication fork progressing in a clockwise direction will encounter the terC site first and pause. If the fork progressing from the anticlockwise direction meets the clockwise fork while paused, replication is terminated, however if it does not meet its anti-fork it will proceed until it reaches the next termination site, terB, where it will pause again, etc [8]. Therefore multiple ter sites are important as infrequently utilized backups, to ensure that the fork does not leave the terminus region, and that termination is completed. Multiple regions to entrap the replication fork means that if an inactivating mutation arises within a ter site, then arrest can still occur at another ter sequence [6].
Replication Terminator Protein (Bacillus subtilis)
| |||||||||||
The Terminus Utilization Substance (Escherichia coli )
| |||||||||||
Biological Significance
The role of the replication fork arrest was primarily believed to be of great importance for the faithful termination of replication, segregation of chromosomes and faithful inheritance of a stable genome. However recent studies where the rtp and tus genes of B.subtilis and E.coli, respectively, were knocked out, suggested that this role is dispensable. Indeed, bacterial systems that have mutations within these genes can survive in the environment and appear identical in both growth rate and cell morphology compared to wildtype bacteria, suggesting that replication termination is not a requirement for cytokinesis [4]. It has recently been suggested that this form of termination may have roles in aiding the co-ordination and optimization of recombination events preceding replication in bacteria, and preventing over-replication. It is also suggested that termination may occur by specific dif sites, conserved sites that are located near the terminus region that are involved in homologous recombination. In fact the dif-terminus hypothesis proposes that termination occurs at or near these sites, where after termination of the replication forks, the dif-sites would undergo site-specific recombination, and that this would resolve the dimer chromosomes and complete replication