Group:MUZIC:Myopodin
From Proteopedia

| Line 11: | Line 11: | ||
A newly identified[[ filamin-binding region within the myopodin molecule]], by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001). | A newly identified[[ filamin-binding region within the myopodin molecule]], by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001). | ||
The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin (Vakeel, 2006). | The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin (Vakeel, 2006). | ||
| - | [[Image:Myopodin | + | [[Image:Myopodin.jpg]] [[Image:Myopodin as interction Partner of FLNC.jpg]] [[Image:MYO PDZ BD.pdb]] |
Revision as of 09:26, 24 June 2011
|
Myopodin it’s a protein encoded by the gene SYNPO2[1], and is widely expressed in striated and smooth muscle cell, but seems to be differentially modified in different tissues. The gene encoding Myopodin protein its form from 6 exon and codify for a protein of 80 kDa was detected in skeletal muscle, whereas the corresponding protein in the heart had a size of 95 kDa, the cause of this size difference remains to be established, but for several reasons, it appears to be due to post translational modifications (Weins et al. 2001)(Linneman et al. JCB 2010).
In undifferentiated myoblasts, myopodin is expressed preferentially in the nucleus and only weakly in the cytoplasm. In differentiated myotubes it is incorporated into the Z-disc and shows no detectable nuclear expression. Notably, this redistribution coincides with an increase in protein expression. Together, these findings indicate that myopodin may be involved in the regulation of myocyte differentiation (Weins et al. 2001).
Myopodin Interactions
Actin was the first binding partner of synaptopodin to be identified (Mundel et al. 1997) and is probably the most important partner. Myopodin has a novel actin binding site (Weins et al. 2001) that was identified by producing truncated fragments from myopodin. The smallest fragment that bound to F-actin contained residues 410–563 of mouse myopodin.
Myopodin binds to α-actinin. That interaction has been shown to involve the spectrin filament domain repeat region of α-actinin (Pham and Chalovich 2006). Synaptopodin family members might be involved in the organization and anchoring of actin in the cell and might be necessary for the correct localization of α-actinin. That is supported by recent findings that myopodin expression precedes α-actinin expression (Linnemann et al. 2010).
A newly identified filamin-binding region within the myopodin molecule, by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001).
The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin (Vakeel, 2006).
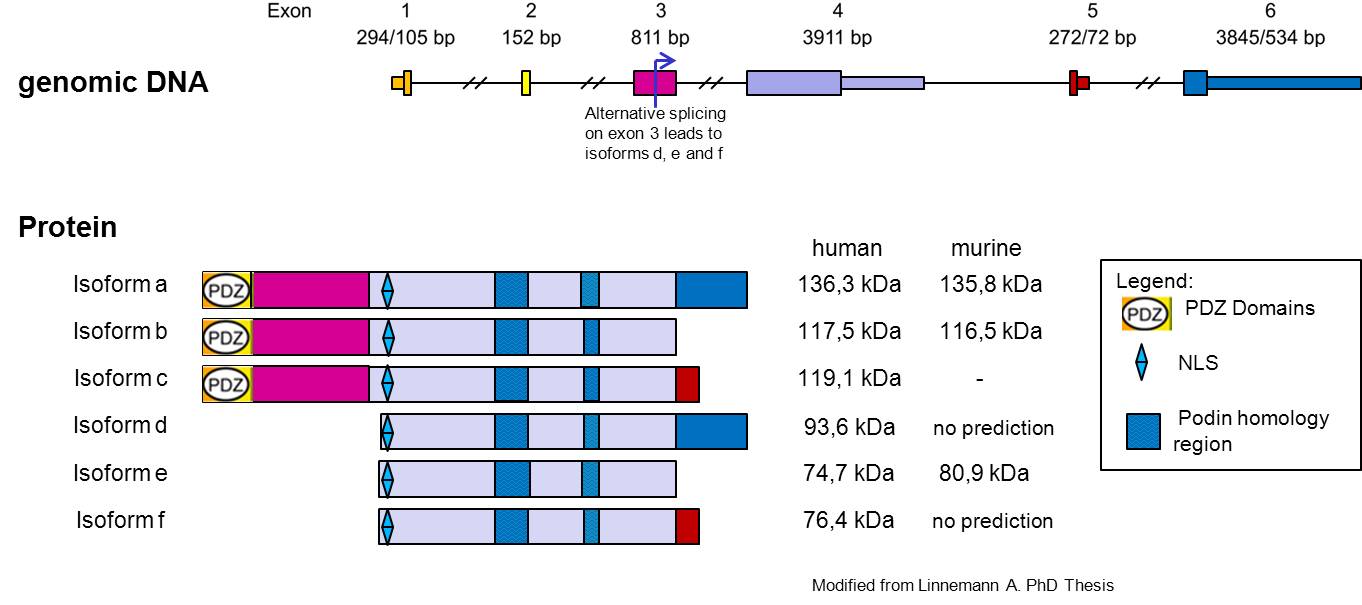 Image:Myopodin as interction Partner of FLNC.jpg Image:MYO PDZ BD.pdb
Image:Myopodin as interction Partner of FLNC.jpg Image:MYO PDZ BD.pdb
