Group:MUZIC:Myopodin
From Proteopedia

| Line 17: | Line 17: | ||
Myopodin binds to α-actinin. That interaction has been shown to involve the spectrin filament domain repeat region of α-actinin (Pham and Chalovich 2006) <ref> PMID:16450054 </ref>. Synaptopodin family members might be involved in the organization and anchoring of actin in the cell and might be necessary for the correct localization of α-actinin. That is supported by recent findings that myopodin expression precedes α-actinin expression (Linnemann et al. 2010). | Myopodin binds to α-actinin. That interaction has been shown to involve the spectrin filament domain repeat region of α-actinin (Pham and Chalovich 2006) <ref> PMID:16450054 </ref>. Synaptopodin family members might be involved in the organization and anchoring of actin in the cell and might be necessary for the correct localization of α-actinin. That is supported by recent findings that myopodin expression precedes α-actinin expression (Linnemann et al. 2010). | ||
A newly identified filamin-binding region within the molecule, by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001) <ref> PMID:11696420 </ref>. | A newly identified filamin-binding region within the molecule, by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001) <ref> PMID:11696420 </ref>. | ||
| - | The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin <ref> PMID:16631741 </ref> | + | The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin (Vakeel, 2006) <ref> PMID:16631741 </ref>. Performing yeast two-hybrid analysis system, Yu and Luo found that myopodin interacts with zyxin both in vitro and in vivo and that this interaction leads to slower migration of prostate cancer cells and reduced invasiveness (Yu YP, Luo JH, 2006) <ref> PMID:16885336 </ref>. |
In the Cardiomyocytes myopodin forms a Z-disc signaling complex with α-actinin, calcineurin, Ca2+/calmodulin-dependent kinase II (CaMKII), muscle-specific A-kinase anchoring protein, and myomegalin. Calcineurin keeps myopodin dephosphorylated, and 14-3-3ß cannot directly bind to myopodin. Upon activation of PKA/CaMKII or inhibition of calcineurin, myopodin undergoes phosphorylation, thereby enabling its interaction with 14-3-3ß, which in turn causes the release of myopodin from Z-disc-anchoring proteins like α-actinin and induces its nuclear import. This novel intracellular signaling pathway, where myopodin was identified as a direct target of PKA, CaMKII, and calcineurin suggest that changes in Z-disc dynamics may translate into compartmentalized signal transduction in the heart (Faul et al. 2007) <ref> PMID:17923693 </ref>. | In the Cardiomyocytes myopodin forms a Z-disc signaling complex with α-actinin, calcineurin, Ca2+/calmodulin-dependent kinase II (CaMKII), muscle-specific A-kinase anchoring protein, and myomegalin. Calcineurin keeps myopodin dephosphorylated, and 14-3-3ß cannot directly bind to myopodin. Upon activation of PKA/CaMKII or inhibition of calcineurin, myopodin undergoes phosphorylation, thereby enabling its interaction with 14-3-3ß, which in turn causes the release of myopodin from Z-disc-anchoring proteins like α-actinin and induces its nuclear import. This novel intracellular signaling pathway, where myopodin was identified as a direct target of PKA, CaMKII, and calcineurin suggest that changes in Z-disc dynamics may translate into compartmentalized signal transduction in the heart (Faul et al. 2007) <ref> PMID:17923693 </ref>. | ||
| + | |||
[[Image:Myopodin interaction with FLNC.jpg|700px]] | [[Image:Myopodin interaction with FLNC.jpg|700px]] | ||
[[Image:Model for the regulation of myopodin's subcellular localization in cardiac myocytes.jpg|500px]] | [[Image:Model for the regulation of myopodin's subcellular localization in cardiac myocytes.jpg|500px]] | ||
Revision as of 18:14, 3 July 2011
|
Contents |
MYOPODIN
Myopodin protein, encoded by the gene SYNPO2[1], also called genethonin-2, synaptopodin-2 and fesselin, was first describe in 2001 by Weins et al., is widely expressed in striated- and smooth-muscle cells (Weins et al., 2001; Nelander et al., 2003) [1] [2] and several reports described the expression of alternative myopodin isoforms in other human cells or tissues (De Ganck et al., 2008; Lin et al., 2001; Sanchez-Carbayo et al., 2003; Schroeter et al., 2008) [3] [4] [5] [6].
Function
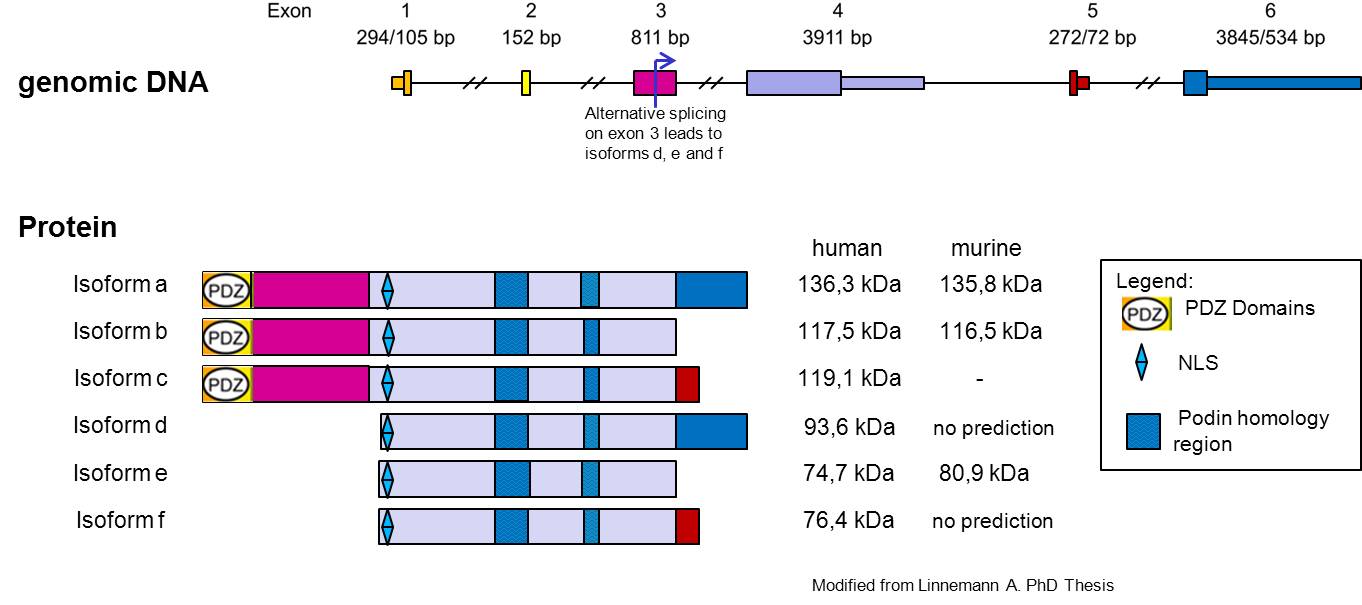
Myopodin is a dual compartment protein that displays actin-bundling activity and redistributes between the nucleus and the cytoplasm in a differentiation-dependent and stress-induced fashion. The gene encoding Myopodin protein its form from 6 exons and codify for a protein of 80 kDa in skeletal muscle and 95kDa in the heart, the cause of this size difference remains to be established, but for several reasons, it appears to be due to post-translational modifications. Beyond the first 3 identified Isoforms, another 3 isoforms have been recently identify (Linneman et al. EJCB 2010) [7]. In undifferentiated myoblasts, myopodin is expressed preferentially in the nucleus and only weakly in the cytoplasm. In differentiated myotubes it is incorporated into the Z-disc and shows no detectable nuclear expression. Notably, this redistribution coincides with an increase in protein expression. Together, these findings indicate that myopodin may be involved in the regulation of myocyte differentiation (Weins et al. 2001). Myopodin gene was identified as a tumor suppressor gene that is frequently deleted in aggressive prostate cancer. Expression of myopodin protein suppresses both tumor growth and metastasis in vitro and in vivo (Jing et al, 2004) [8].
Myopodin Interactions
Actin was the first binding partner of synaptopodin to be identified (Mundel et al. 1997) [9] and is probably the most important partner. Myopodin has a novel actin binding site (Weins et al. 2001) that was identified by producing truncated fragments from myopodin. The smallest fragment that bound to F-actin contained residues 410–563 of mouse myopodin. Myopodin binds to α-actinin. That interaction has been shown to involve the spectrin filament domain repeat region of α-actinin (Pham and Chalovich 2006) [10]. Synaptopodin family members might be involved in the organization and anchoring of actin in the cell and might be necessary for the correct localization of α-actinin. That is supported by recent findings that myopodin expression precedes α-actinin expression (Linnemann et al. 2010). A newly identified filamin-binding region within the molecule, by performing yeast two-hybrid assays using carboxy-terminally and/or amino-terminally truncated constructs (Linnemann et al. 2010). The interaction was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin (Lin et al., 2001) [11]. The alternative transcription offers the possibility of expression of two isoforms of Myopodin, which probably differ in their binding properties for these PDZ binding domain detected, however, there is preliminary evidence that the PDZ binding domain from Myopodin interacts with C terminal part of Synemin (Vakeel, 2006) [12]. Performing yeast two-hybrid analysis system, Yu and Luo found that myopodin interacts with zyxin both in vitro and in vivo and that this interaction leads to slower migration of prostate cancer cells and reduced invasiveness (Yu YP, Luo JH, 2006) [13]. In the Cardiomyocytes myopodin forms a Z-disc signaling complex with α-actinin, calcineurin, Ca2+/calmodulin-dependent kinase II (CaMKII), muscle-specific A-kinase anchoring protein, and myomegalin. Calcineurin keeps myopodin dephosphorylated, and 14-3-3ß cannot directly bind to myopodin. Upon activation of PKA/CaMKII or inhibition of calcineurin, myopodin undergoes phosphorylation, thereby enabling its interaction with 14-3-3ß, which in turn causes the release of myopodin from Z-disc-anchoring proteins like α-actinin and induces its nuclear import. This novel intracellular signaling pathway, where myopodin was identified as a direct target of PKA, CaMKII, and calcineurin suggest that changes in Z-disc dynamics may translate into compartmentalized signal transduction in the heart (Faul et al. 2007) [14].
Image:Myopodin interaction with FLNC.jpg Image:Model for the regulation of myopodin's subcellular localization in cardiac myocytes.jpg
References
- ↑ Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol. 2001 Oct 29;155(3):393-404. Epub 2001 Oct 22. PMID:11673475 doi:10.1083/jcb.200012039
- ↑ Nelander S, Mostad P, Lindahl P. Prediction of cell type-specific gene modules: identification and initial characterization of a core set of smooth muscle-specific genes. Genome Res. 2003 Aug;13(8):1838-54. Epub 2003 Jul 17. PMID:12869577 doi:10.1101/gr.1197303
- ↑ De Ganck A, De Corte V, Staes A, Gevaert K, Vandekerckhove J, Gettemans J. Multiple isoforms of the tumor suppressor myopodin are simultaneously transcribed in cancer cells. Biochem Biophys Res Commun. 2008 May 30;370(2):269-73. Epub 2008 Mar 25. PMID:18371299 doi:10.1016/j.bbrc.2008.03.086
- ↑ Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, Dhir R, Krill D, Becich MJ, Michalopoulos G, Finkelstein S, Luo JH. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am J Pathol. 2001 Nov;159(5):1603-12. PMID:11696420 doi:10.1016/S0002-9440(10)63006-4
- ↑ Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003 Aug 14;22(34):5298-305. PMID:12917631 doi:http://dx.doi.org/10.1038/sj.onc.1206616
- ↑ Schroeter MM, Beall B, Heid HW, Chalovich JM. In vitro characterization of native mammalian smooth-muscle protein synaptopodin 2. Biosci Rep. 2008 Aug;28(4):195-203. PMID:18588515 doi:10.1042/BSR20080079
- ↑ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010 Sep;89(9):681-92. Epub 2010 May 31. PMID:20554076 doi:10.1016/j.ejcb.2010.04.004
- ↑ Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, Cieply K, Wells A, Luo JH. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004 May;164(5):1799-806. PMID:15111326 doi:10.1016/S0002-9440(10)63738-8
- ↑ Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997 Oct 6;139(1):193-204. PMID:9314539
- ↑ Pham M, Chalovich JM. Smooth muscle alpha-actinin binds tightly to fesselin and attenuates its activity toward actin polymerization. J Muscle Res Cell Motil. 2006;27(1):45-51. Epub 2006 Feb 1. PMID:16450054 doi:10.1007/s10974-005-9053-2
- ↑ Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, Dhir R, Krill D, Becich MJ, Michalopoulos G, Finkelstein S, Luo JH. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am J Pathol. 2001 Nov;159(5):1603-12. PMID:11696420 doi:10.1016/S0002-9440(10)63006-4
- ↑ van der Ven PF, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B, Furst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp Cell Res. 2006 Jul 1;312(11):2154-67. Epub 2006 Apr 24. PMID:16631741 doi:S0014-4827(06)00110-8
- ↑ Yu YP, Luo JH. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Res. 2006 Aug 1;66(15):7414-9. PMID:16885336 doi:10.1158/0008-5472.CAN-06-0227
- ↑ Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007 Dec;27(23):8215-27. Epub 2007 Oct 8. PMID:17923693 doi:10.1128/MCB.00950-07